Input interpretation
barium chromate(V) | copper(II) molybdate
Chemical names and formulas
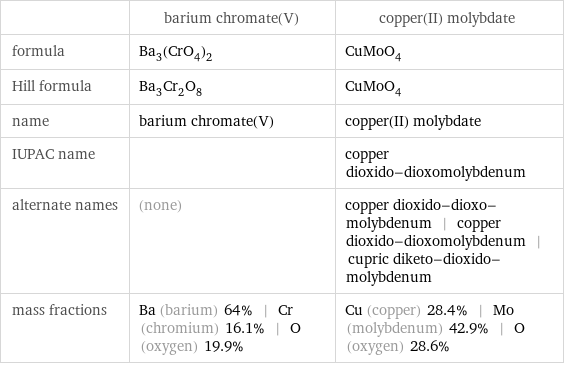
| barium chromate(V) | copper(II) molybdate formula | Ba_3(CrO_4)_2 | CuMoO_4 Hill formula | Ba_3Cr_2O_8 | CuMoO_4 name | barium chromate(V) | copper(II) molybdate IUPAC name | | copper dioxido-dioxomolybdenum alternate names | (none) | copper dioxido-dioxo-molybdenum | copper dioxido-dioxomolybdenum | cupric diketo-dioxido-molybdenum mass fractions | Ba (barium) 64% | Cr (chromium) 16.1% | O (oxygen) 19.9% | Cu (copper) 28.4% | Mo (molybdenum) 42.9% | O (oxygen) 28.6%
Structure diagrams
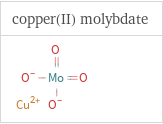
Structure diagrams
Basic properties
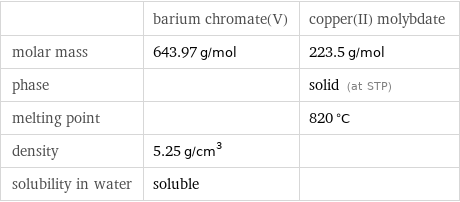
| barium chromate(V) | copper(II) molybdate molar mass | 643.97 g/mol | 223.5 g/mol phase | | solid (at STP) melting point | | 820 °C density | 5.25 g/cm^3 | solubility in water | soluble |
Units
