Input interpretation
vanadium(II) oxide | lead(II) phthalocyanine
Chemical names and formulas
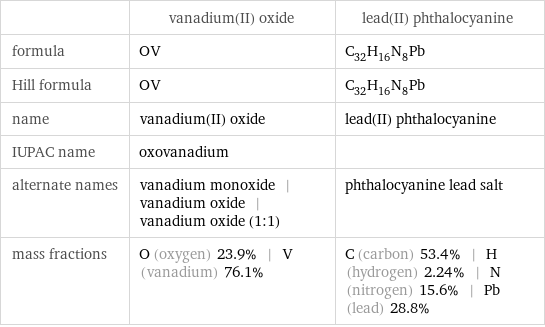
| vanadium(II) oxide | lead(II) phthalocyanine formula | OV | C_32H_16N_8Pb Hill formula | OV | C_32H_16N_8Pb name | vanadium(II) oxide | lead(II) phthalocyanine IUPAC name | oxovanadium | alternate names | vanadium monoxide | vanadium oxide | vanadium oxide (1:1) | phthalocyanine lead salt mass fractions | O (oxygen) 23.9% | V (vanadium) 76.1% | C (carbon) 53.4% | H (hydrogen) 2.24% | N (nitrogen) 15.6% | Pb (lead) 28.8%
Structure diagrams
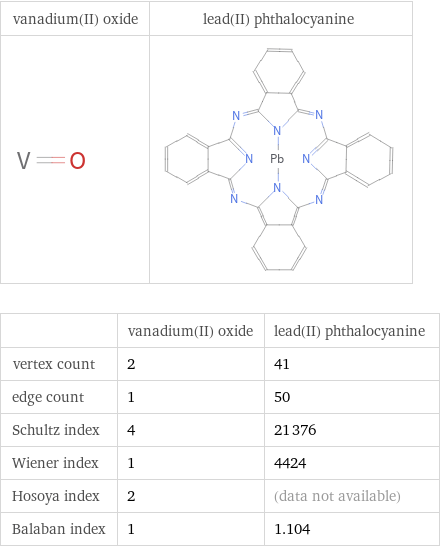
| vanadium(II) oxide | lead(II) phthalocyanine vertex count | 2 | 41 edge count | 1 | 50 Schultz index | 4 | 21376 Wiener index | 1 | 4424 Hosoya index | 2 | (data not available) Balaban index | 1 | 1.104
Basic properties
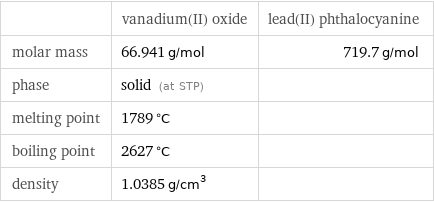
| vanadium(II) oxide | lead(II) phthalocyanine molar mass | 66.941 g/mol | 719.7 g/mol phase | solid (at STP) | melting point | 1789 °C | boiling point | 2627 °C | density | 1.0385 g/cm^3 |
Units

Thermodynamic properties
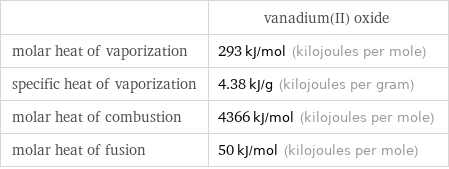
| vanadium(II) oxide molar heat of vaporization | 293 kJ/mol (kilojoules per mole) specific heat of vaporization | 4.38 kJ/g (kilojoules per gram) molar heat of combustion | 4366 kJ/mol (kilojoules per mole) molar heat of fusion | 50 kJ/mol (kilojoules per mole)