Input interpretation

alkali metals | molar heat capacity
Summary
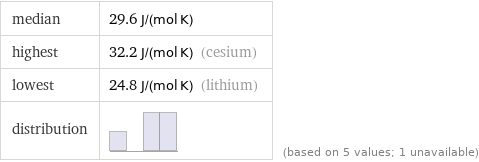
median | 29.6 J/(mol K) highest | 32.2 J/(mol K) (cesium) lowest | 24.8 J/(mol K) (lithium) distribution | | (based on 5 values; 1 unavailable)
Entity with missing value
francium
Ranked values
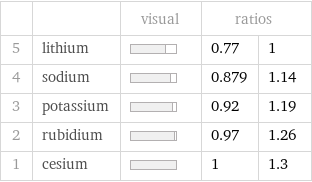
| | visual | ratios | 5 | lithium | | 0.77 | 1 4 | sodium | | 0.879 | 1.14 3 | potassium | | 0.92 | 1.19 2 | rubidium | | 0.97 | 1.26 1 | cesium | | 1 | 1.3
Molar heat capacity rankings

1 | lithium | 24.8 J/(mol K) 2 | sodium | 28.28 J/(mol K) 3 | potassium | 29.6 J/(mol K) 4 | rubidium | 31.1 J/(mol K) 5 | cesium | 32.2 J/(mol K) (based on 5 values; 1 unavailable)
Unit conversions for median molar heat capacity 29.6 J/(mol K)
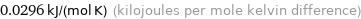
0.0296 kJ/(mol K) (kilojoules per mole kelvin difference)
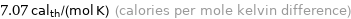
7.07 cal_th/(mol K) (calories per mole kelvin difference)
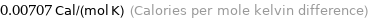
0.00707 Cal/(mol K) (Calories per mole kelvin difference)