Input interpretation

group 10 elements | phase at STP
Results
nickel | solid palladium | solid platinum | solid darmstadtium | (data not available)

group 10 elements | phase at STP | solid | solid | solid (all) (based on 3 values; 1 unavailable)
Thermodynamic properties
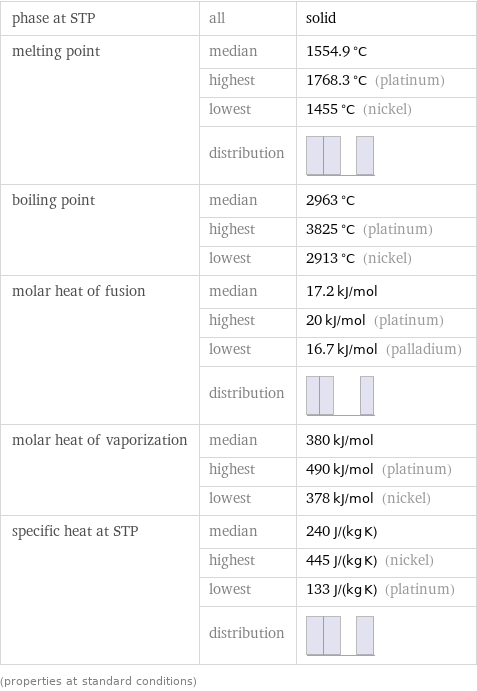
phase at STP | all | solid melting point | median | 1554.9 °C | highest | 1768.3 °C (platinum) | lowest | 1455 °C (nickel) | distribution | boiling point | median | 2963 °C | highest | 3825 °C (platinum) | lowest | 2913 °C (nickel) molar heat of fusion | median | 17.2 kJ/mol | highest | 20 kJ/mol (platinum) | lowest | 16.7 kJ/mol (palladium) | distribution | molar heat of vaporization | median | 380 kJ/mol | highest | 490 kJ/mol (platinum) | lowest | 378 kJ/mol (nickel) specific heat at STP | median | 240 J/(kg K) | highest | 445 J/(kg K) (nickel) | lowest | 133 J/(kg K) (platinum) | distribution | (properties at standard conditions)