Input interpretation

manganese(II) nitrate hydrate | vanadium trihydroxide
Chemical names and formulas
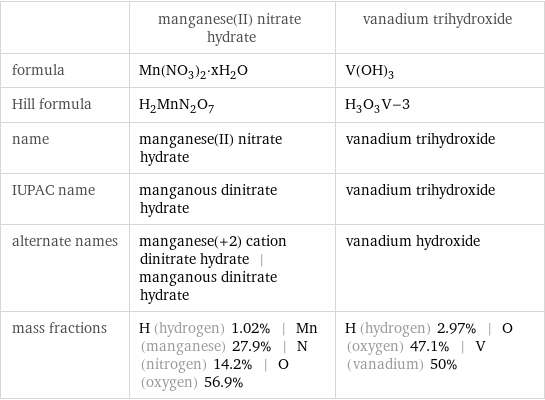
| manganese(II) nitrate hydrate | vanadium trihydroxide formula | Mn(NO_3)_2·xH_2O | V(OH)_3 Hill formula | H_2MnN_2O_7 | H_3O_3V-3 name | manganese(II) nitrate hydrate | vanadium trihydroxide IUPAC name | manganous dinitrate hydrate | vanadium trihydroxide alternate names | manganese(+2) cation dinitrate hydrate | manganous dinitrate hydrate | vanadium hydroxide mass fractions | H (hydrogen) 1.02% | Mn (manganese) 27.9% | N (nitrogen) 14.2% | O (oxygen) 56.9% | H (hydrogen) 2.97% | O (oxygen) 47.1% | V (vanadium) 50%
Structure diagrams
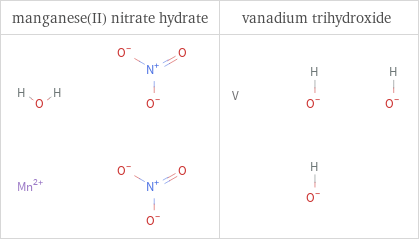
Structure diagrams
Basic properties

| manganese(II) nitrate hydrate | vanadium trihydroxide molar mass | 196.96 g/mol | 101.96 g/mol
Units

Chemical identifiers
([O-])[O-].[N+](=O)([O-])[O-].O.[Mn+2] | [OH-].[OH-].[OH-].[V]](../image_source/8eb47b9953142e87270fdb75ec2606d7.png)
| manganese(II) nitrate hydrate | vanadium trihydroxide CAS number | 15710-66-4 | 59865-92-8 PubChem CID number | 16211480 | 3017159 PubChem SID number | 24852196 | SMILES identifier | [N+](=O)([O-])[O-].[N+](=O)([O-])[O-].O.[Mn+2] | [OH-].[OH-].[OH-].[V]