Input interpretation

bismuth | number of valence electrons
Result
![Determine the number of valence electrons in: bismuth (Bi) Plan: • Look up the abbreviated (condensed) ground electronic configuration. • Use the electronic configuration to identify the outermost electrons. • Use the principal quantum number to identify the valence electrons. The abbreviated ground electronic configuration for bismuth is: [Xe]6s^24f^145d^106p^3 Electrons in atomic orbitals outside of the xenon core, [Xe], are the outermost electrons: 6s^24f^145d^106p^3 Extract the principal quantum number, n, for the outermost electrons from the electron configuration: electron configuration | n | orbital | number of electrons 6s^2 | 6 | s | 2 4f^14 | 4 | f | 14 5d^10 | 5 | d | 10 6p^3 | 6 | p | 3 The outermost electrons with the largest principal quantum number (in this case, n = 6) are valence electrons. So the number of valence electrons in bismuth is: Answer: | | 2 + 3 = 5](../image_source/364f791120d35644b2561f1799d1d21b.png)
Determine the number of valence electrons in: bismuth (Bi) Plan: • Look up the abbreviated (condensed) ground electronic configuration. • Use the electronic configuration to identify the outermost electrons. • Use the principal quantum number to identify the valence electrons. The abbreviated ground electronic configuration for bismuth is: [Xe]6s^24f^145d^106p^3 Electrons in atomic orbitals outside of the xenon core, [Xe], are the outermost electrons: 6s^24f^145d^106p^3 Extract the principal quantum number, n, for the outermost electrons from the electron configuration: electron configuration | n | orbital | number of electrons 6s^2 | 6 | s | 2 4f^14 | 4 | f | 14 5d^10 | 5 | d | 10 6p^3 | 6 | p | 3 The outermost electrons with the largest principal quantum number (in this case, n = 6) are valence electrons. So the number of valence electrons in bismuth is: Answer: | | 2 + 3 = 5
Corresponding quantity
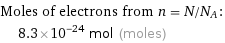
Moles of electrons from n = N/N_A: | 8.3×10^-24 mol (moles)