Input interpretation
non-metallic elements
Members
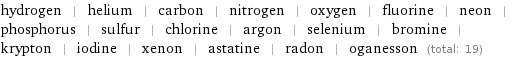
hydrogen | helium | carbon | nitrogen | oxygen | fluorine | neon | phosphorus | sulfur | chlorine | argon | selenium | bromine | krypton | iodine | xenon | astatine | radon | oganesson (total: 19)
Periodic table location
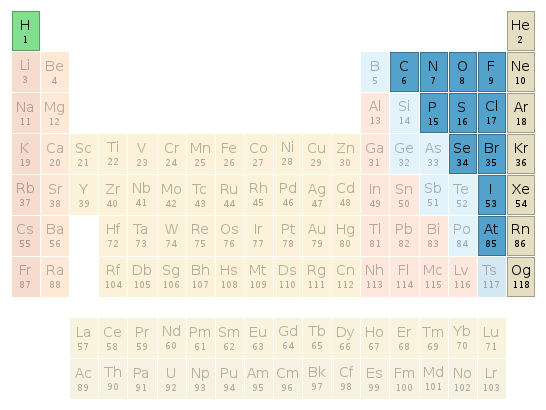
Periodic table location
Images

Images
Basic elemental properties
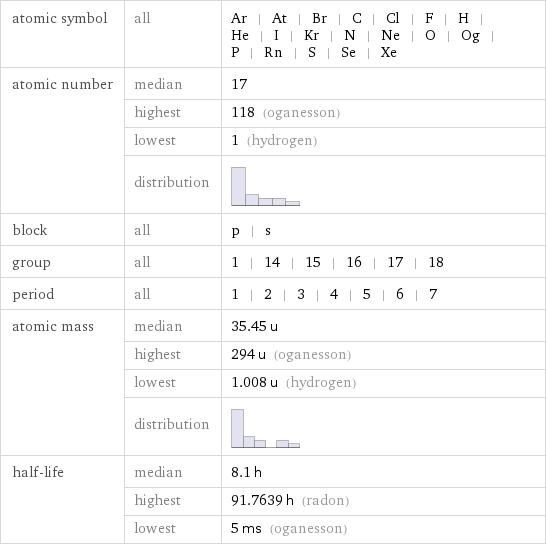
atomic symbol | all | Ar | At | Br | C | Cl | F | H | He | I | Kr | N | Ne | O | Og | P | Rn | S | Se | Xe atomic number | median | 17 | highest | 118 (oganesson) | lowest | 1 (hydrogen) | distribution | block | all | p | s group | all | 1 | 14 | 15 | 16 | 17 | 18 period | all | 1 | 2 | 3 | 4 | 5 | 6 | 7 atomic mass | median | 35.45 u | highest | 294 u (oganesson) | lowest | 1.008 u (hydrogen) | distribution | half-life | median | 8.1 h | highest | 91.7639 h (radon) | lowest | 5 ms (oganesson)
Thermodynamic properties
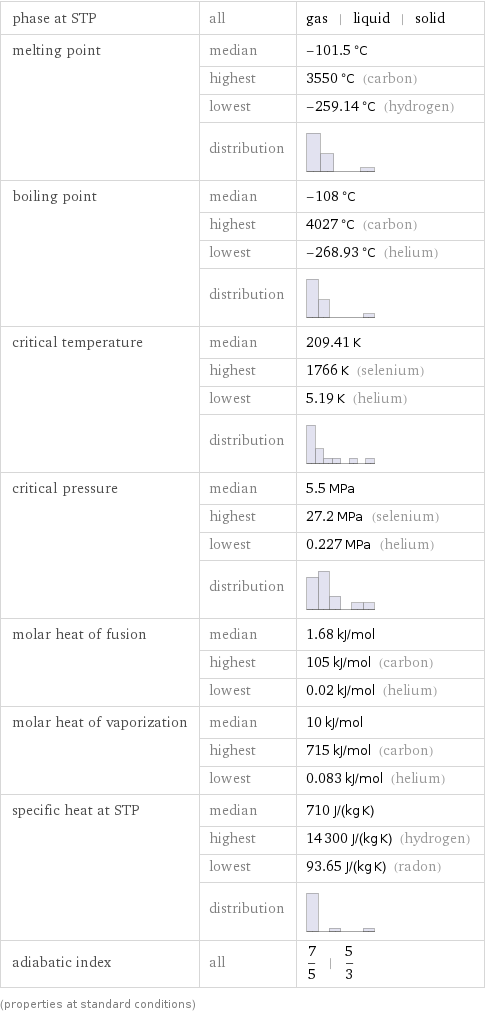
phase at STP | all | gas | liquid | solid melting point | median | -101.5 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | -108 °C | highest | 4027 °C (carbon) | lowest | -268.93 °C (helium) | distribution | critical temperature | median | 209.41 K | highest | 1766 K (selenium) | lowest | 5.19 K (helium) | distribution | critical pressure | median | 5.5 MPa | highest | 27.2 MPa (selenium) | lowest | 0.227 MPa (helium) | distribution | molar heat of fusion | median | 1.68 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.02 kJ/mol (helium) molar heat of vaporization | median | 10 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 0.083 kJ/mol (helium) specific heat at STP | median | 710 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 93.65 J/(kg K) (radon) | distribution | adiabatic index | all | 7/5 | 5/3 (properties at standard conditions)
Material properties
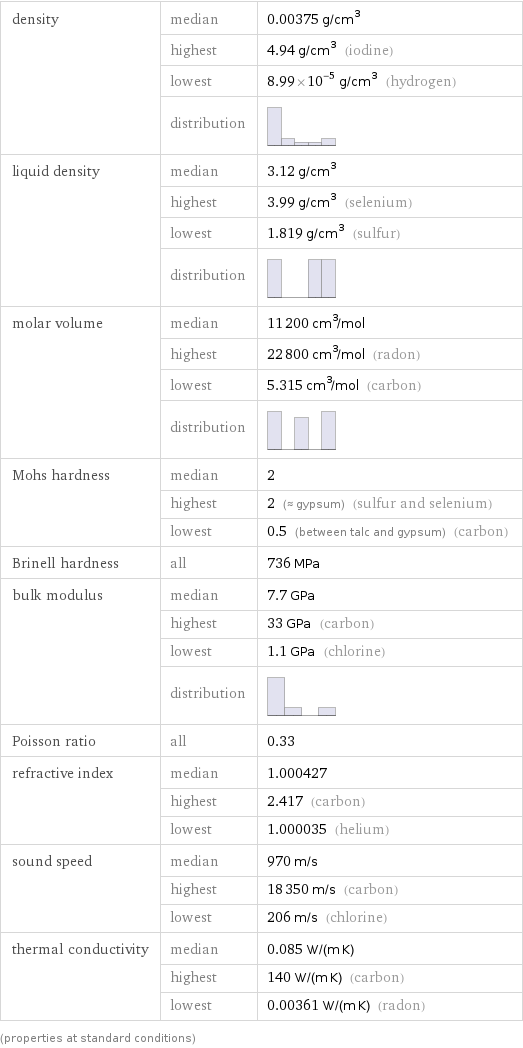
density | median | 0.00375 g/cm^3 | highest | 4.94 g/cm^3 (iodine) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 3.12 g/cm^3 | highest | 3.99 g/cm^3 (selenium) | lowest | 1.819 g/cm^3 (sulfur) | distribution | molar volume | median | 11200 cm^3/mol | highest | 22800 cm^3/mol (radon) | lowest | 5.315 cm^3/mol (carbon) | distribution | Mohs hardness | median | 2 | highest | 2 (≈ gypsum) (sulfur and selenium) | lowest | 0.5 (between talc and gypsum) (carbon) Brinell hardness | all | 736 MPa bulk modulus | median | 7.7 GPa | highest | 33 GPa (carbon) | lowest | 1.1 GPa (chlorine) | distribution | Poisson ratio | all | 0.33 refractive index | median | 1.000427 | highest | 2.417 (carbon) | lowest | 1.000035 (helium) sound speed | median | 970 m/s | highest | 18350 m/s (carbon) | lowest | 206 m/s (chlorine) thermal conductivity | median | 0.085 W/(m K) | highest | 140 W/(m K) (carbon) | lowest | 0.00361 W/(m K) (radon) (properties at standard conditions)
Electromagnetic properties
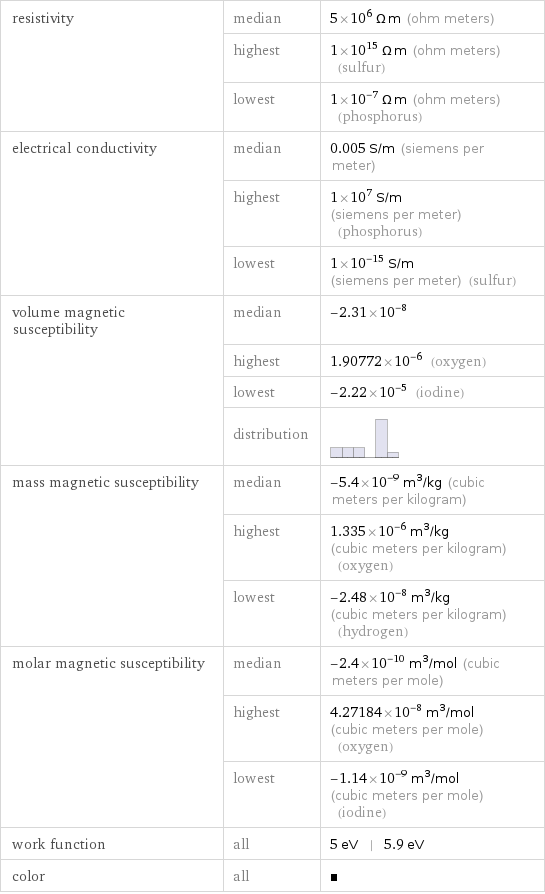
resistivity | median | 5×10^6 Ω m (ohm meters) | highest | 1×10^15 Ω m (ohm meters) (sulfur) | lowest | 1×10^-7 Ω m (ohm meters) (phosphorus) electrical conductivity | median | 0.005 S/m (siemens per meter) | highest | 1×10^7 S/m (siemens per meter) (phosphorus) | lowest | 1×10^-15 S/m (siemens per meter) (sulfur) volume magnetic susceptibility | median | -2.31×10^-8 | highest | 1.90772×10^-6 (oxygen) | lowest | -2.22×10^-5 (iodine) | distribution | mass magnetic susceptibility | median | -5.4×10^-9 m^3/kg (cubic meters per kilogram) | highest | 1.335×10^-6 m^3/kg (cubic meters per kilogram) (oxygen) | lowest | -2.48×10^-8 m^3/kg (cubic meters per kilogram) (hydrogen) molar magnetic susceptibility | median | -2.4×10^-10 m^3/mol (cubic meters per mole) | highest | 4.27184×10^-8 m^3/mol (cubic meters per mole) (oxygen) | lowest | -1.14×10^-9 m^3/mol (cubic meters per mole) (iodine) work function | all | 5 eV | 5.9 eV color | all |
Reactivity
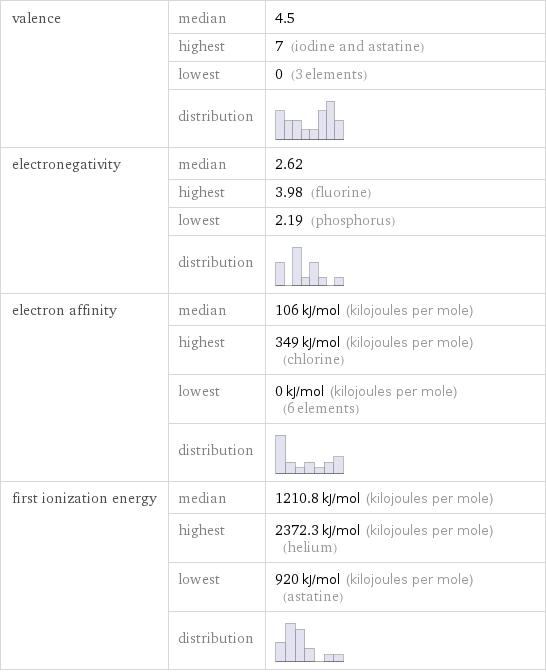
valence | median | 4.5 | highest | 7 (iodine and astatine) | lowest | 0 (3 elements) | distribution | electronegativity | median | 2.62 | highest | 3.98 (fluorine) | lowest | 2.19 (phosphorus) | distribution | electron affinity | median | 106 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 0 kJ/mol (kilojoules per mole) (6 elements) | distribution | first ionization energy | median | 1210.8 kJ/mol (kilojoules per mole) | highest | 2372.3 kJ/mol (kilojoules per mole) (helium) | lowest | 920 kJ/mol (kilojoules per mole) (astatine) | distribution |