Input interpretation
hydrogen bromide | sodium bromate
Chemical names and formulas
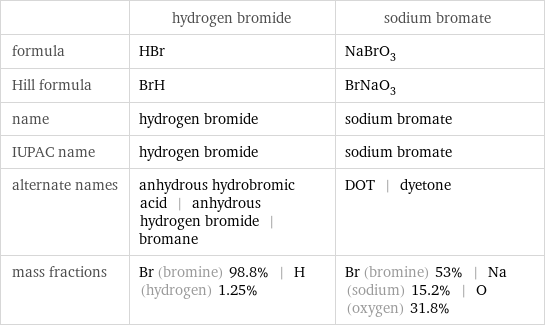
| hydrogen bromide | sodium bromate formula | HBr | NaBrO_3 Hill formula | BrH | BrNaO_3 name | hydrogen bromide | sodium bromate IUPAC name | hydrogen bromide | sodium bromate alternate names | anhydrous hydrobromic acid | anhydrous hydrogen bromide | bromane | DOT | dyetone mass fractions | Br (bromine) 98.8% | H (hydrogen) 1.25% | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8%
Structure diagrams
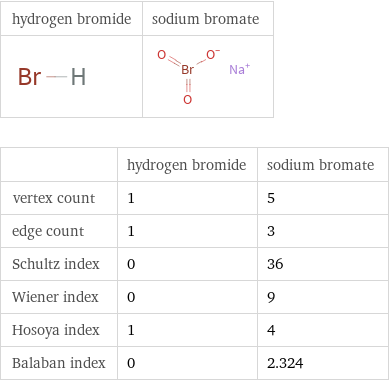
| hydrogen bromide | sodium bromate vertex count | 1 | 5 edge count | 1 | 3 Schultz index | 0 | 36 Wiener index | 0 | 9 Hosoya index | 1 | 4 Balaban index | 0 | 2.324
3D structure
3D structure
Basic properties
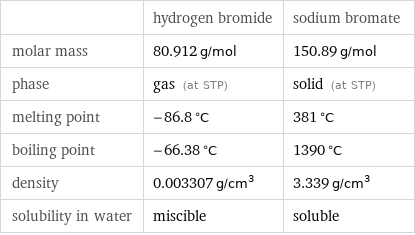
| hydrogen bromide | sodium bromate molar mass | 80.912 g/mol | 150.89 g/mol phase | gas (at STP) | solid (at STP) melting point | -86.8 °C | 381 °C boiling point | -66.38 °C | 1390 °C density | 0.003307 g/cm^3 | 3.339 g/cm^3 solubility in water | miscible | soluble
Units

Gas properties
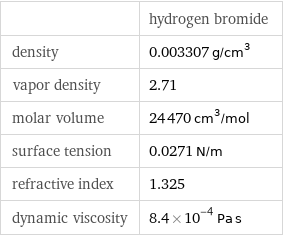
| hydrogen bromide density | 0.003307 g/cm^3 vapor density | 2.71 molar volume | 24470 cm^3/mol surface tension | 0.0271 N/m refractive index | 1.325 dynamic viscosity | 8.4×10^-4 Pa s
Units

Liquid properties

| hydrogen bromide sound speed | 720 km/h
Units

Thermodynamic properties
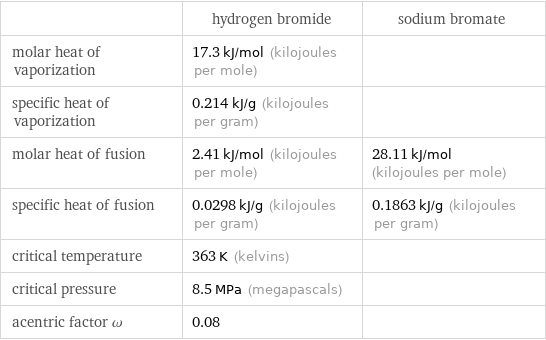
| hydrogen bromide | sodium bromate molar heat of vaporization | 17.3 kJ/mol (kilojoules per mole) | specific heat of vaporization | 0.214 kJ/g (kilojoules per gram) | molar heat of fusion | 2.41 kJ/mol (kilojoules per mole) | 28.11 kJ/mol (kilojoules per mole) specific heat of fusion | 0.0298 kJ/g (kilojoules per gram) | 0.1863 kJ/g (kilojoules per gram) critical temperature | 363 K (kelvins) | critical pressure | 8.5 MPa (megapascals) | acentric factor ω | 0.08 |
Solid properties
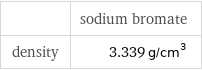
| sodium bromate density | 3.339 g/cm^3
Units
