Input interpretation
americium | tetrabutylphosphonium bromide
Chemical names and formulas
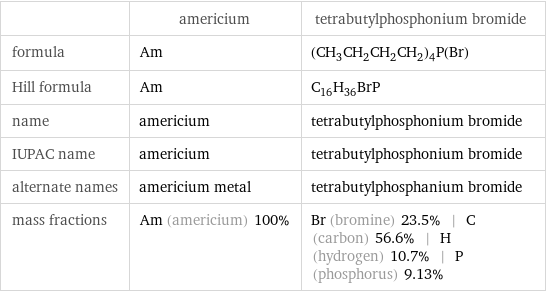
| americium | tetrabutylphosphonium bromide formula | Am | (CH_3CH_2CH_2CH_2)_4P(Br) Hill formula | Am | C_16H_36BrP name | americium | tetrabutylphosphonium bromide IUPAC name | americium | tetrabutylphosphonium bromide alternate names | americium metal | tetrabutylphosphanium bromide mass fractions | Am (americium) 100% | Br (bromine) 23.5% | C (carbon) 56.6% | H (hydrogen) 10.7% | P (phosphorus) 9.13%
Structure diagrams
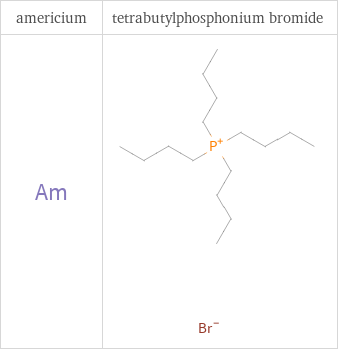
Structure diagrams
Basic properties
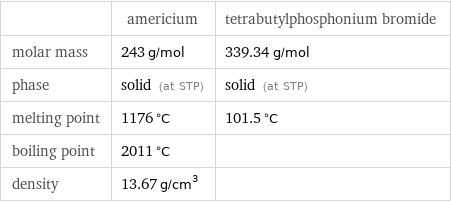
| americium | tetrabutylphosphonium bromide molar mass | 243 g/mol | 339.34 g/mol phase | solid (at STP) | solid (at STP) melting point | 1176 °C | 101.5 °C boiling point | 2011 °C | density | 13.67 g/cm^3 |
Units
