Input interpretation
ethylene dichloride
Chemical names and formulas
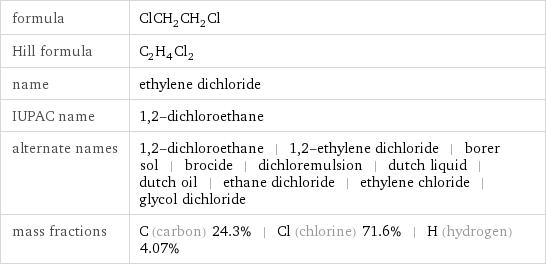
formula | ClCH_2CH_2Cl Hill formula | C_2H_4Cl_2 name | ethylene dichloride IUPAC name | 1, 2-dichloroethane alternate names | 1, 2-dichloroethane | 1, 2-ethylene dichloride | borer sol | brocide | dichloremulsion | dutch liquid | dutch oil | ethane dichloride | ethylene chloride | glycol dichloride mass fractions | C (carbon) 24.3% | Cl (chlorine) 71.6% | H (hydrogen) 4.07%
Lewis structure
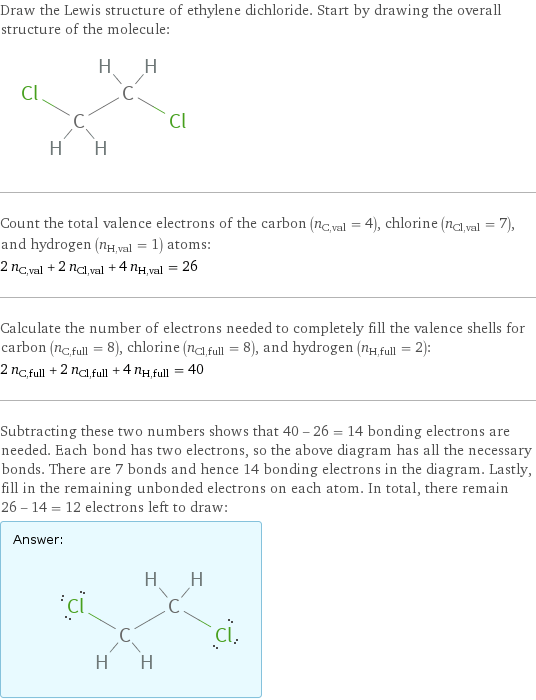
Draw the Lewis structure of ethylene dichloride. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), and hydrogen (n_H, val = 1) atoms: 2 n_C, val + 2 n_Cl, val + 4 n_H, val = 26 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), and hydrogen (n_H, full = 2): 2 n_C, full + 2 n_Cl, full + 4 n_H, full = 40 Subtracting these two numbers shows that 40 - 26 = 14 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 7 bonds and hence 14 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 26 - 14 = 12 electrons left to draw: Answer: | |
3D structure
3D structure
Basic properties
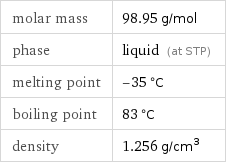
molar mass | 98.95 g/mol phase | liquid (at STP) melting point | -35 °C boiling point | 83 °C density | 1.256 g/cm^3
Units

Hydrophobicity and permeability properties
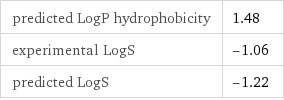
predicted LogP hydrophobicity | 1.48 experimental LogS | -1.06 predicted LogS | -1.22
Basic drug properties
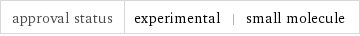
approval status | experimental | small molecule
Liquid properties (at STP)
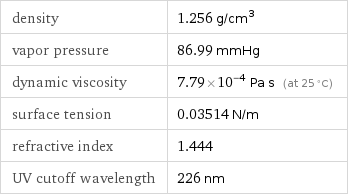
density | 1.256 g/cm^3 vapor pressure | 86.99 mmHg dynamic viscosity | 7.79×10^-4 Pa s (at 25 °C) surface tension | 0.03514 N/m refractive index | 1.444 UV cutoff wavelength | 226 nm
Units

Thermodynamic properties
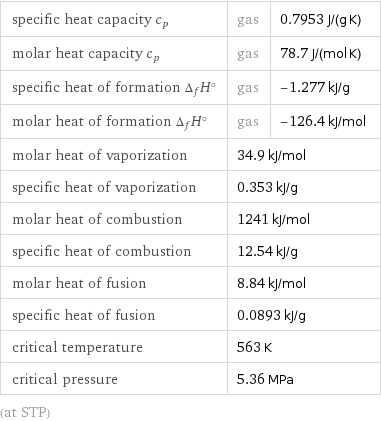
specific heat capacity c_p | gas | 0.7953 J/(g K) molar heat capacity c_p | gas | 78.7 J/(mol K) specific heat of formation Δ_fH° | gas | -1.277 kJ/g molar heat of formation Δ_fH° | gas | -126.4 kJ/mol molar heat of vaporization | 34.9 kJ/mol | specific heat of vaporization | 0.353 kJ/g | molar heat of combustion | 1241 kJ/mol | specific heat of combustion | 12.54 kJ/g | molar heat of fusion | 8.84 kJ/mol | specific heat of fusion | 0.0893 kJ/g | critical temperature | 563 K | critical pressure | 5.36 MPa | (at STP)
Chemical identifiers
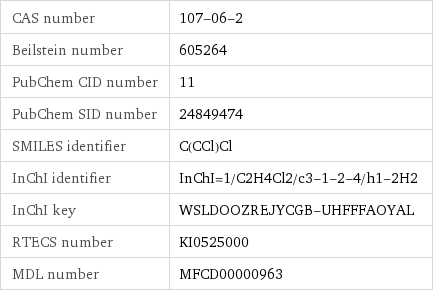
CAS number | 107-06-2 Beilstein number | 605264 PubChem CID number | 11 PubChem SID number | 24849474 SMILES identifier | C(CCl)Cl InChI identifier | InChI=1/C2H4Cl2/c3-1-2-4/h1-2H2 InChI key | WSLDOOZREJYCGB-UHFFFAOYAL RTECS number | KI0525000 MDL number | MFCD00000963
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 3 NFPA reactivity rating | 0