Input interpretation

ytterbium (chemical element) | actinium (chemical element) | europium (chemical element)
Periodic table location

Periodic table location
Images
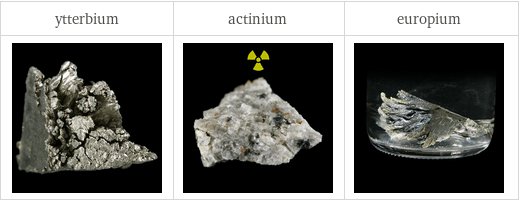
Images
Basic elemental properties
![| ytterbium | actinium | europium atomic symbol | Yb | Ac | Eu atomic number | 70 | 89 | 63 short electronic configuration | [Xe]6s^24f^14 | [Rn]7s^26d^1 | [Xe]6s^24f^7 Aufbau diagram | 4f 6s | 6d 7s | 4f 6s block | f | f | f period | 6 | 7 | 6 atomic mass | 173.045 u | 227 u | 151.964 u half-life | (stable) | 21.787 yr | (stable)](../image_source/dbe86ae806734713609112b582fb4ad7.png)
| ytterbium | actinium | europium atomic symbol | Yb | Ac | Eu atomic number | 70 | 89 | 63 short electronic configuration | [Xe]6s^24f^14 | [Rn]7s^26d^1 | [Xe]6s^24f^7 Aufbau diagram | 4f 6s | 6d 7s | 4f 6s block | f | f | f period | 6 | 7 | 6 atomic mass | 173.045 u | 227 u | 151.964 u half-life | (stable) | 21.787 yr | (stable)
Thermodynamic properties
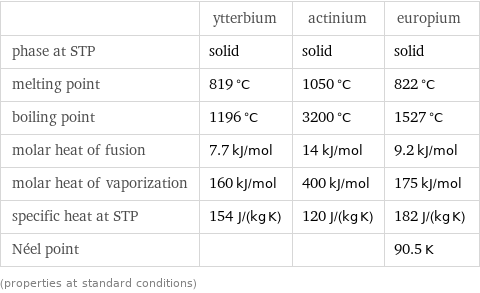
| ytterbium | actinium | europium phase at STP | solid | solid | solid melting point | 819 °C | 1050 °C | 822 °C boiling point | 1196 °C | 3200 °C | 1527 °C molar heat of fusion | 7.7 kJ/mol | 14 kJ/mol | 9.2 kJ/mol molar heat of vaporization | 160 kJ/mol | 400 kJ/mol | 175 kJ/mol specific heat at STP | 154 J/(kg K) | 120 J/(kg K) | 182 J/(kg K) Néel point | | | 90.5 K (properties at standard conditions)
Material properties
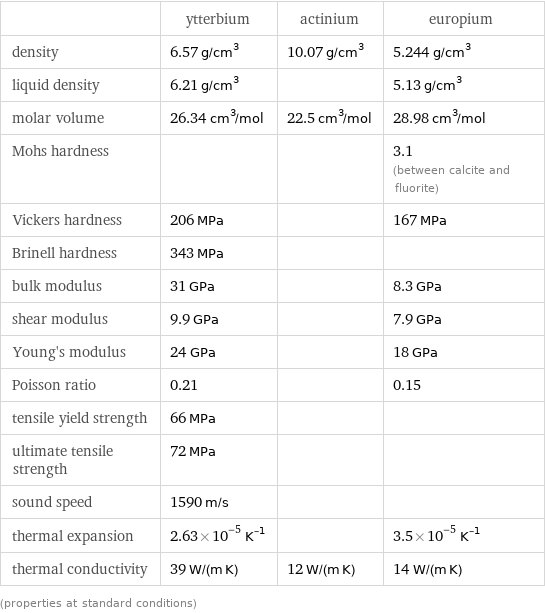
| ytterbium | actinium | europium density | 6.57 g/cm^3 | 10.07 g/cm^3 | 5.244 g/cm^3 liquid density | 6.21 g/cm^3 | | 5.13 g/cm^3 molar volume | 26.34 cm^3/mol | 22.5 cm^3/mol | 28.98 cm^3/mol Mohs hardness | | | 3.1 (between calcite and fluorite) Vickers hardness | 206 MPa | | 167 MPa Brinell hardness | 343 MPa | | bulk modulus | 31 GPa | | 8.3 GPa shear modulus | 9.9 GPa | | 7.9 GPa Young's modulus | 24 GPa | | 18 GPa Poisson ratio | 0.21 | | 0.15 tensile yield strength | 66 MPa | | ultimate tensile strength | 72 MPa | | sound speed | 1590 m/s | | thermal expansion | 2.63×10^-5 K^(-1) | | 3.5×10^-5 K^(-1) thermal conductivity | 39 W/(m K) | 12 W/(m K) | 14 W/(m K) (properties at standard conditions)
Electromagnetic properties

| ytterbium | actinium | europium electrical type | conductor | | conductor resistivity | 2.8×10^-7 Ω m (ohm meters) | | 9×10^-7 Ω m (ohm meters) electrical conductivity | 3.6×10^6 S/m (siemens per meter) | | 1.1×10^6 S/m (siemens per meter) magnetic type | paramagnetic | | paramagnetic volume magnetic susceptibility | 3.88×10^-5 | | 0.0014473 mass magnetic susceptibility | 5.9×10^-9 m^3/kg (cubic meters per kilogram) | | 2.76×10^-7 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 1.02×10^-9 m^3/mol (cubic meters per mole) | | 4.1942×10^-8 m^3/mol (cubic meters per mole) Néel point | | | 90.5 K (kelvins) work function | | | 2.5 eV (Polycrystalline) threshold frequency | | | 6.045×10^14 Hz (hertz) superconducting point | | | 1.8 K (kelvins) color | (silver) | (silver) | (silver)
Reactivity

| ytterbium | actinium | europium valence | 3 | 3 | 3 electronegativity | | 1.1 | electron affinity | 50 kJ/mol (kilojoules per mole) | | 50 kJ/mol (kilojoules per mole) first ionization energy | 603.4 kJ/mol (kilojoules per mole) | 499 kJ/mol (kilojoules per mole) | 547.1 kJ/mol (kilojoules per mole) ionization energies | 603.4 kJ/mol | 1174.8 kJ/mol | 2417 kJ/mol | 4203 kJ/mol | 499 kJ/mol | 1170 kJ/mol | 547.1 kJ/mol | 1085 kJ/mol | 2404 kJ/mol | 4120 kJ/mol
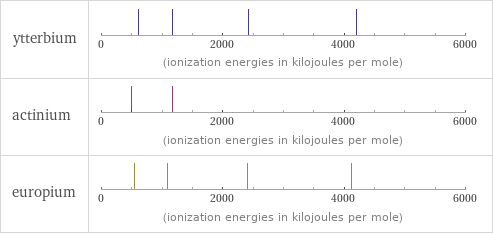
Reactivity
Atomic properties
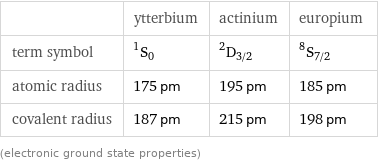
| ytterbium | actinium | europium term symbol | ^1S_0 | ^2D_(3/2) | ^8S_(7/2) atomic radius | 175 pm | 195 pm | 185 pm covalent radius | 187 pm | 215 pm | 198 pm (electronic ground state properties)
Abundances
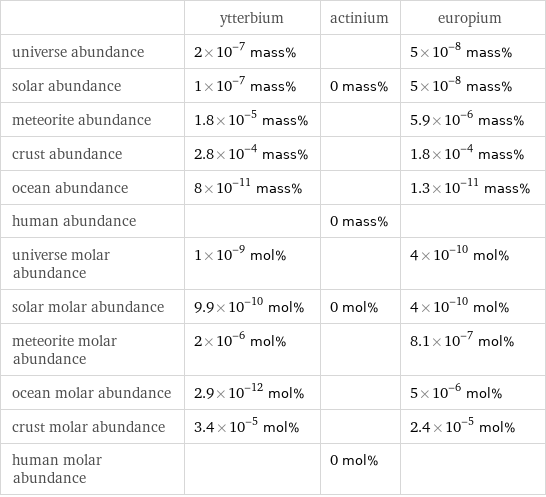
| ytterbium | actinium | europium universe abundance | 2×10^-7 mass% | | 5×10^-8 mass% solar abundance | 1×10^-7 mass% | 0 mass% | 5×10^-8 mass% meteorite abundance | 1.8×10^-5 mass% | | 5.9×10^-6 mass% crust abundance | 2.8×10^-4 mass% | | 1.8×10^-4 mass% ocean abundance | 8×10^-11 mass% | | 1.3×10^-11 mass% human abundance | | 0 mass% | universe molar abundance | 1×10^-9 mol% | | 4×10^-10 mol% solar molar abundance | 9.9×10^-10 mol% | 0 mol% | 4×10^-10 mol% meteorite molar abundance | 2×10^-6 mol% | | 8.1×10^-7 mol% ocean molar abundance | 2.9×10^-12 mol% | | 5×10^-6 mol% crust molar abundance | 3.4×10^-5 mol% | | 2.4×10^-5 mol% human molar abundance | | 0 mol% |