Input interpretation
cadmium chloride monohydrate | tellurium
Chemical names and formulas
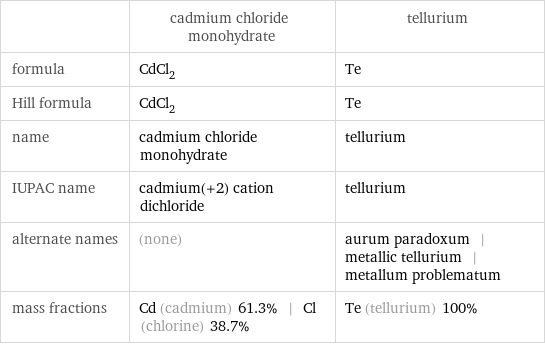
| cadmium chloride monohydrate | tellurium formula | CdCl_2 | Te Hill formula | CdCl_2 | Te name | cadmium chloride monohydrate | tellurium IUPAC name | cadmium(+2) cation dichloride | tellurium alternate names | (none) | aurum paradoxum | metallic tellurium | metallum problematum mass fractions | Cd (cadmium) 61.3% | Cl (chlorine) 38.7% | Te (tellurium) 100%
Structure diagrams

Structure diagrams
Basic properties
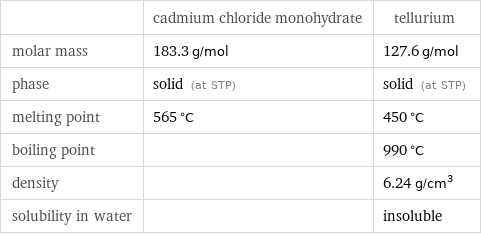
| cadmium chloride monohydrate | tellurium molar mass | 183.3 g/mol | 127.6 g/mol phase | solid (at STP) | solid (at STP) melting point | 565 °C | 450 °C boiling point | | 990 °C density | | 6.24 g/cm^3 solubility in water | | insoluble
Units

Thermodynamic properties
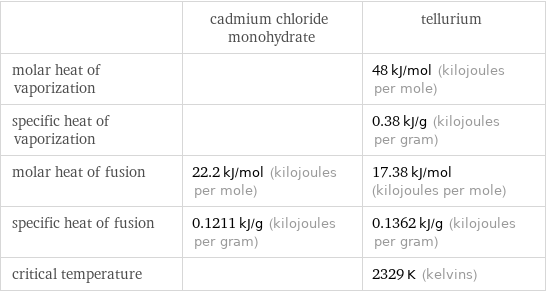
| cadmium chloride monohydrate | tellurium molar heat of vaporization | | 48 kJ/mol (kilojoules per mole) specific heat of vaporization | | 0.38 kJ/g (kilojoules per gram) molar heat of fusion | 22.2 kJ/mol (kilojoules per mole) | 17.38 kJ/mol (kilojoules per mole) specific heat of fusion | 0.1211 kJ/g (kilojoules per gram) | 0.1362 kJ/g (kilojoules per gram) critical temperature | | 2329 K (kelvins)
Solid properties (at STP)
| tellurium density | 6.24 g/cm^3 vapor pressure | 3.22929×10^-6 mmHg refractive index | 1.000991
Units
