Input interpretation
hydronium cation | chloride anion | nitrate anion
Structure diagrams
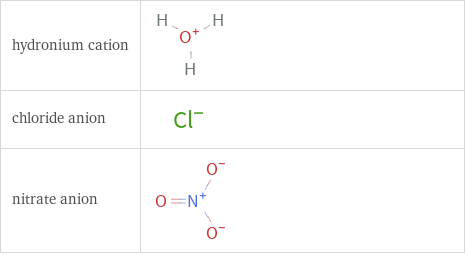
Structure diagrams
General properties
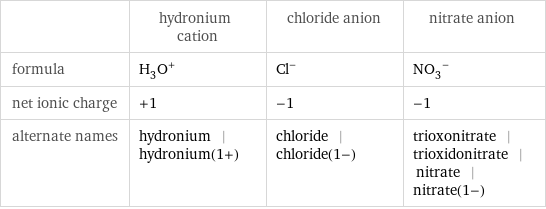
| hydronium cation | chloride anion | nitrate anion formula | (H_3O)^+ | Cl^- | (NO_3)^- net ionic charge | +1 | -1 | -1 alternate names | hydronium | hydronium(1+) | chloride | chloride(1-) | trioxonitrate | trioxidonitrate | nitrate | nitrate(1-)
Ionic radii

| chloride anion | nitrate anion 6-coordinate ionic radius | 184 pm | thermochemical radius | | 179 pm
Units

Other properties
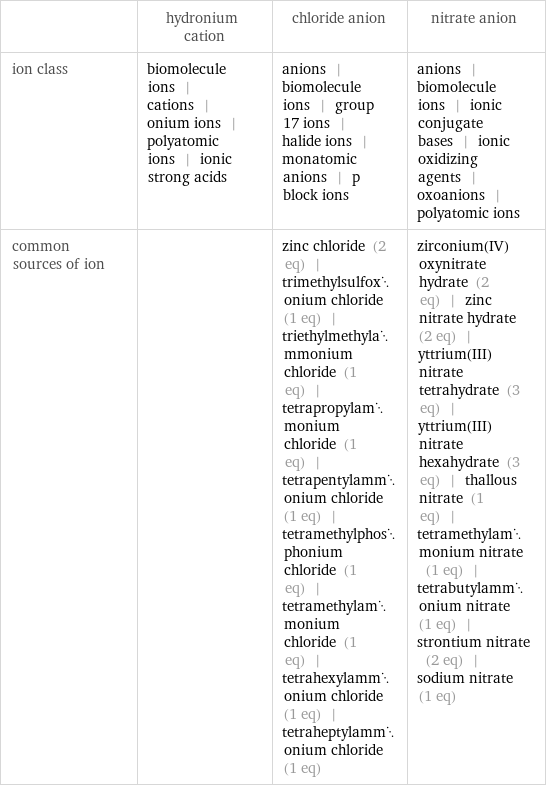
| hydronium cation | chloride anion | nitrate anion ion class | biomolecule ions | cations | onium ions | polyatomic ions | ionic strong acids | anions | biomolecule ions | group 17 ions | halide ions | monatomic anions | p block ions | anions | biomolecule ions | ionic conjugate bases | ionic oxidizing agents | oxoanions | polyatomic ions common sources of ion | | zinc chloride (2 eq) | trimethylsulfoxonium chloride (1 eq) | triethylmethylammonium chloride (1 eq) | tetrapropylammonium chloride (1 eq) | tetrapentylammonium chloride (1 eq) | tetramethylphosphonium chloride (1 eq) | tetramethylammonium chloride (1 eq) | tetrahexylammonium chloride (1 eq) | tetraheptylammonium chloride (1 eq) | zirconium(IV) oxynitrate hydrate (2 eq) | zinc nitrate hydrate (2 eq) | yttrium(III)nitrate tetrahydrate (3 eq) | yttrium(III) nitrate hexahydrate (3 eq) | thallous nitrate (1 eq) | tetramethylammonium nitrate (1 eq) | tetrabutylammonium nitrate (1 eq) | strontium nitrate (2 eq) | sodium nitrate (1 eq)