Input interpretation

perchlorate anion
Lewis structure
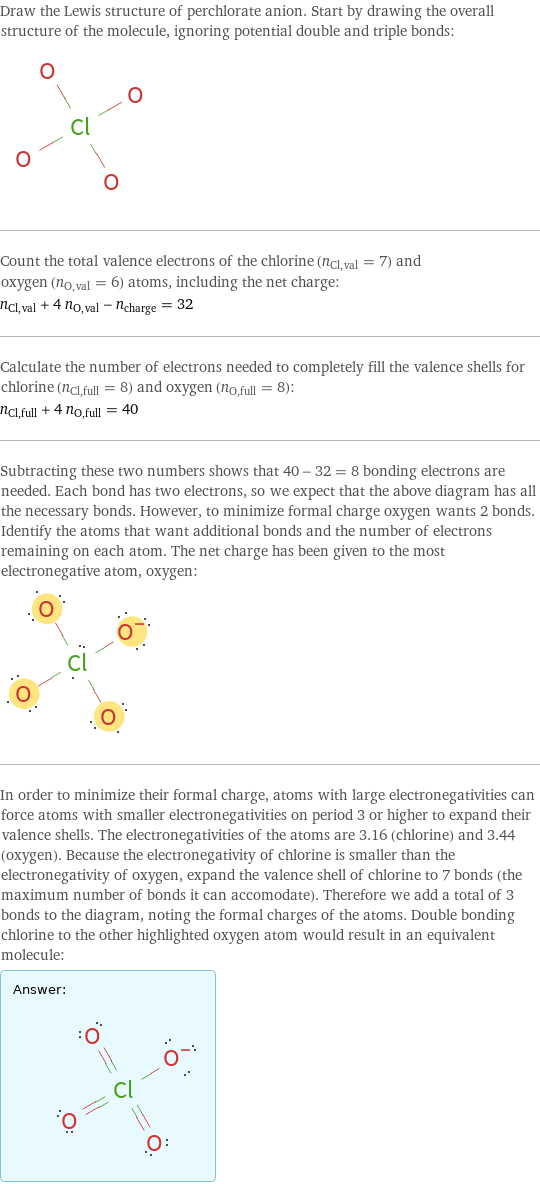
Draw the Lewis structure of perchlorate anion. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the chlorine (n_Cl, val = 7) and oxygen (n_O, val = 6) atoms, including the net charge: n_Cl, val + 4 n_O, val - n_charge = 32 Calculate the number of electrons needed to completely fill the valence shells for chlorine (n_Cl, full = 8) and oxygen (n_O, full = 8): n_Cl, full + 4 n_O, full = 40 Subtracting these two numbers shows that 40 - 32 = 8 bonding electrons are needed. Each bond has two electrons, so we expect that the above diagram has all the necessary bonds. However, to minimize formal charge oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom. The net charge has been given to the most electronegative atom, oxygen: In order to minimize their formal charge, atoms with large electronegativities can force atoms with smaller electronegativities on period 3 or higher to expand their valence shells. The electronegativities of the atoms are 3.16 (chlorine) and 3.44 (oxygen). Because the electronegativity of chlorine is smaller than the electronegativity of oxygen, expand the valence shell of chlorine to 7 bonds (the maximum number of bonds it can accomodate). Therefore we add a total of 3 bonds to the diagram, noting the formal charges of the atoms. Double bonding chlorine to the other highlighted oxygen atom would result in an equivalent molecule: Answer: | |
General properties

formula | (ClO_4)^- net ionic charge | -1 alternate names | tetraoxochlorate | tetraoxidochlorate | perchlorate | perchlorate(1-)
Ionic radius

thermochemical radius | 240 pm
Units

Other properties
![ion class | anions | oxoanions | polyatomic ions common sources of ion | thallium(III) perchlorate hydrate (3 eq) | sodium perchlorate (1 eq) | silver perchlorate (1 eq) | rubidium perchlorate (1 eq) | potassium perchlorate (1 eq) | 5, 9-bis(ethylamino)-10-methylbenzo[a]phenoxazin-7-ium perchlorate (1 eq)](../image_source/f66d6555123dc0ef978b96704f356df5.png)
ion class | anions | oxoanions | polyatomic ions common sources of ion | thallium(III) perchlorate hydrate (3 eq) | sodium perchlorate (1 eq) | silver perchlorate (1 eq) | rubidium perchlorate (1 eq) | potassium perchlorate (1 eq) | 5, 9-bis(ethylamino)-10-methylbenzo[a]phenoxazin-7-ium perchlorate (1 eq)
Thermodynamic properties
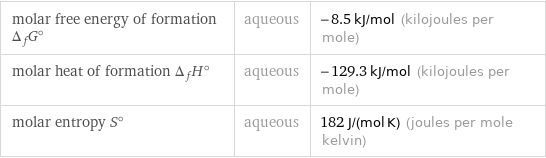
molar free energy of formation Δ_fG° | aqueous | -8.5 kJ/mol (kilojoules per mole) molar heat of formation Δ_fH° | aqueous | -129.3 kJ/mol (kilojoules per mole) molar entropy S° | aqueous | 182 J/(mol K) (joules per mole kelvin)