Input interpretation

period 3 elements | van der Waals radius
Summary

median | 180 pm highest | 227 pm (sodium) lowest | 173 pm (magnesium) distribution | | (based on 7 values; 1 unavailable)
Entity with missing value
aluminum
Ranked values
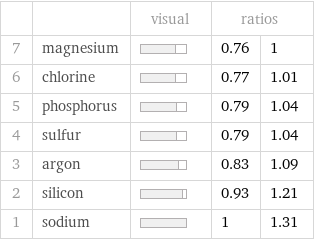
| | visual | ratios | 7 | magnesium | | 0.76 | 1 6 | chlorine | | 0.77 | 1.01 5 | phosphorus | | 0.79 | 1.04 4 | sulfur | | 0.79 | 1.04 3 | argon | | 0.83 | 1.09 2 | silicon | | 0.93 | 1.21 1 | sodium | | 1 | 1.31
Plot
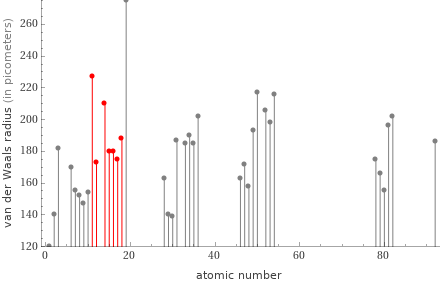
Plot
Periodic table location
Periodic table location
Atomic radii properties
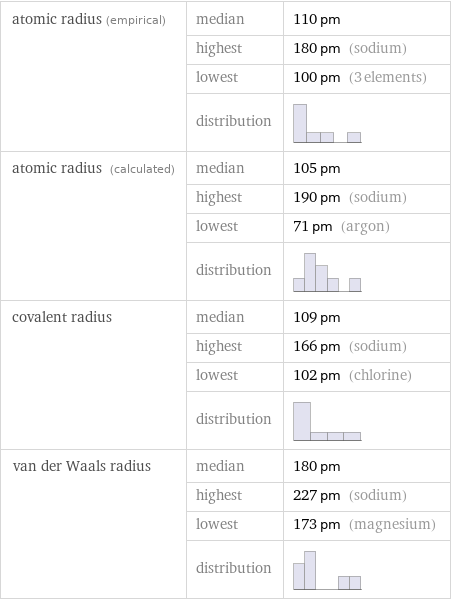
atomic radius (empirical) | median | 110 pm | highest | 180 pm (sodium) | lowest | 100 pm (3 elements) | distribution | atomic radius (calculated) | median | 105 pm | highest | 190 pm (sodium) | lowest | 71 pm (argon) | distribution | covalent radius | median | 109 pm | highest | 166 pm (sodium) | lowest | 102 pm (chlorine) | distribution | van der Waals radius | median | 180 pm | highest | 227 pm (sodium) | lowest | 173 pm (magnesium) | distribution |
Units

Van der Waals radius rankings
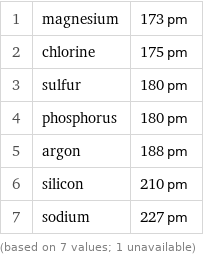
1 | magnesium | 173 pm 2 | chlorine | 175 pm 3 | sulfur | 180 pm 4 | phosphorus | 180 pm 5 | argon | 188 pm 6 | silicon | 210 pm 7 | sodium | 227 pm (based on 7 values; 1 unavailable)
Unit conversions for median van der Waals radius 180 pm
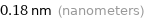
0.18 nm (nanometers)
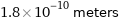
1.8×10^-10 meters
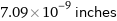
7.09×10^-9 inches
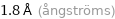
1.8 Å (ångströms)
Comparisons for median van der Waals radius 180 pm
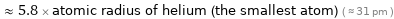
≈ 5.8 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
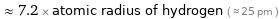
≈ 7.2 × atomic radius of hydrogen ( ≈ 25 pm )