Input interpretation

polar protic solvents | ebullioscopic constant K_b
Summary
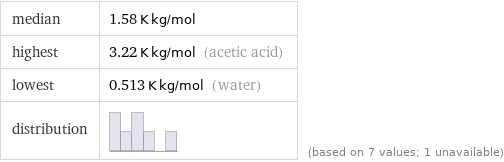
median | 1.58 K kg/mol highest | 3.22 K kg/mol (acetic acid) lowest | 0.513 K kg/mol (water) distribution | | (based on 7 values; 1 unavailable)
Entity with missing value
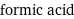
formic acid
Ranked values
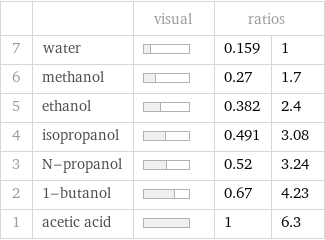
| | visual | ratios | 7 | water | | 0.159 | 1 6 | methanol | | 0.27 | 1.7 5 | ethanol | | 0.382 | 2.4 4 | isopropanol | | 0.491 | 3.08 3 | N-propanol | | 0.52 | 3.24 2 | 1-butanol | | 0.67 | 4.23 1 | acetic acid | | 1 | 6.3
Ebullioscopic constant K_b rankings
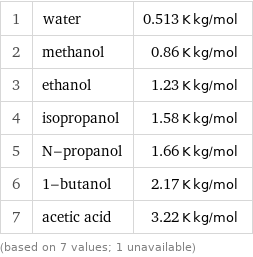
1 | water | 0.513 K kg/mol 2 | methanol | 0.86 K kg/mol 3 | ethanol | 1.23 K kg/mol 4 | isopropanol | 1.58 K kg/mol 5 | N-propanol | 1.66 K kg/mol 6 | 1-butanol | 2.17 K kg/mol 7 | acetic acid | 3.22 K kg/mol (based on 7 values; 1 unavailable)
Unit conversions for median ebullioscopic constant K_b 1.58 K kg/mol
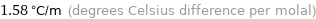
1.58 °C/m (degrees Celsius difference per molal)
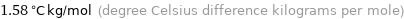
1.58 °C kg/mol (degree Celsius difference kilograms per mole)
Comparisons for median ebullioscopic constant K_b 1.58 K kg/mol

≈ 0.68 × boiling point elevation constant of carbon disulfide ( ≈ 2.3 K kg/mol )

≈ 0.76 × boiling point elevation constant of diethyl ether ( 2 to 2.2 K kg/mol )
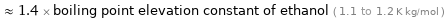
≈ 1.4 × boiling point elevation constant of ethanol ( 1.1 to 1.2 K kg/mol )