Input interpretation
carfentrazone-ethyl
Chemical names and formulas
![formula | C_15H_14Cl_2F_3N_3O_3 name | carfentrazone-ethyl IUPAC name | ethyl 2-chloro-3-[2-chloro-5-[4-(difluoromethyl)-3-methyl-5-oxo-1, 2, 4-triazol-1-yl]-4-fluoro-phenyl]propanoate alternate names | aim | aurora | carfentrazone ethyl | spotlight mass fractions | C (carbon) 43.7% | Cl (chlorine) 17.2% | F (fluorine) 13.8% | H (hydrogen) 3.42% | N (nitrogen) 10.2% | O (oxygen) 11.6%](../image_source/cd574d25c96a03386184c90b512ff500.png)
formula | C_15H_14Cl_2F_3N_3O_3 name | carfentrazone-ethyl IUPAC name | ethyl 2-chloro-3-[2-chloro-5-[4-(difluoromethyl)-3-methyl-5-oxo-1, 2, 4-triazol-1-yl]-4-fluoro-phenyl]propanoate alternate names | aim | aurora | carfentrazone ethyl | spotlight mass fractions | C (carbon) 43.7% | Cl (chlorine) 17.2% | F (fluorine) 13.8% | H (hydrogen) 3.42% | N (nitrogen) 10.2% | O (oxygen) 11.6%
Lewis structure
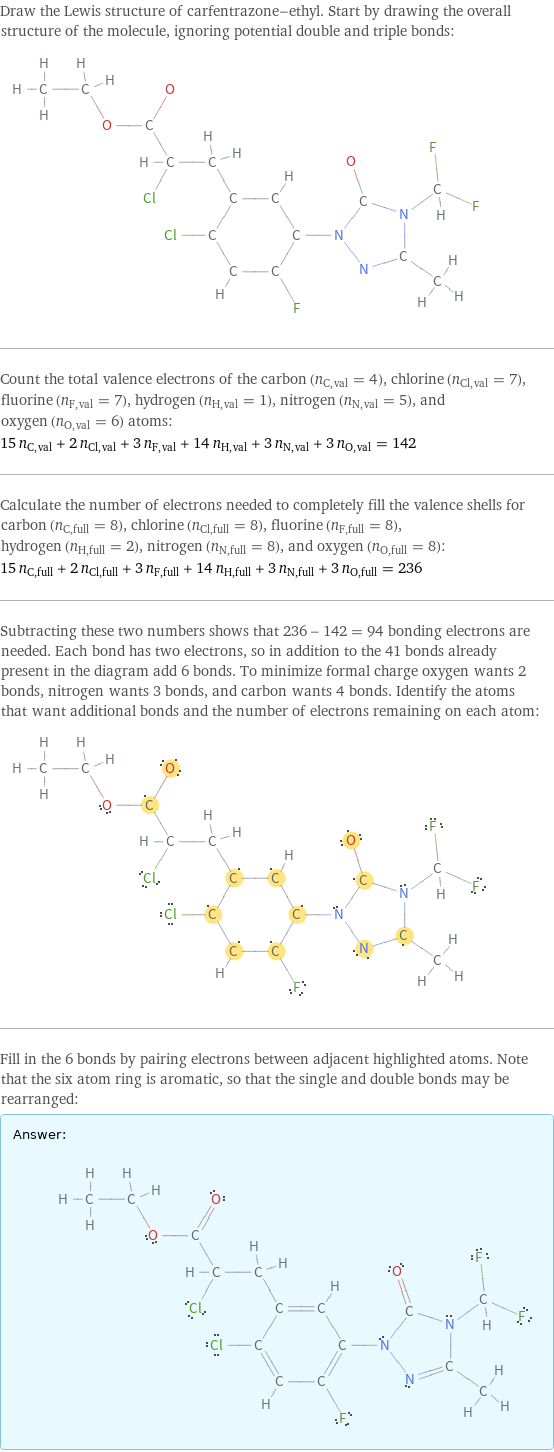
Draw the Lewis structure of carfentrazone-ethyl. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), fluorine (n_F, val = 7), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 15 n_C, val + 2 n_Cl, val + 3 n_F, val + 14 n_H, val + 3 n_N, val + 3 n_O, val = 142 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), fluorine (n_F, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 15 n_C, full + 2 n_Cl, full + 3 n_F, full + 14 n_H, full + 3 n_N, full + 3 n_O, full = 236 Subtracting these two numbers shows that 236 - 142 = 94 bonding electrons are needed. Each bond has two electrons, so in addition to the 41 bonds already present in the diagram add 6 bonds. To minimize formal charge oxygen wants 2 bonds, nitrogen wants 3 bonds, and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 6 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom ring is aromatic, so that the single and double bonds may be rearranged: Answer: | |
3D structure
3D structure
Basic properties
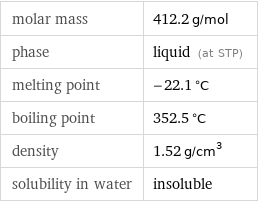
molar mass | 412.2 g/mol phase | liquid (at STP) melting point | -22.1 °C boiling point | 352.5 °C density | 1.52 g/cm^3 solubility in water | insoluble
Units

Liquid properties (at STP)

density | 1.52 g/cm^3 vapor pressure | 2.1×10^-8 mmHg (at 25 °C)
Units

Thermodynamic properties

molar heat of vaporization | 71.3 kJ/mol specific heat of vaporization | 0.173 kJ/g (at STP)
Chemical identifiers

CAS number | 128639-02-1 PubChem CID number | 86222 SMILES identifier | CCOC(=O)C(CC1=CC(=C(C=C1Cl)F)N2C(=O)N(C(=N2)C)C(F)F)Cl InChI identifier | InChI=1/C15H14Cl2F3N3O3/c1-3-26-13(24)10(17)4-8-5-12(11(18)6-9(8)16)23-15(25)22(14(19)20)7(2)21-23/h5-6, 10, 14H, 3-4H2, 1-2H3
Safety properties

flash point | 228 °C

DOT hazard class | 9 DOT numbers | 3077