Input interpretation

plutonium (chemical element)
Periodic table location
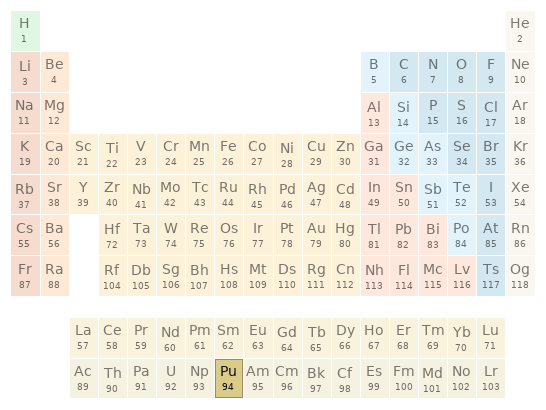
Periodic table location
Image

Image
Basic elemental properties
![atomic symbol | Pu atomic number | 94 short electronic configuration | [Rn]7s^25f^6 Aufbau diagram | 5f 7s block | f period | 7 atomic mass | 244 u (unified atomic mass units) (atomic mass number given for longest-lived isotope) half-life | 79 Myr (megayears)](../image_source/f0c716c1c4281354834f5938d807cfae.png)
atomic symbol | Pu atomic number | 94 short electronic configuration | [Rn]7s^25f^6 Aufbau diagram | 5f 7s block | f period | 7 atomic mass | 244 u (unified atomic mass units) (atomic mass number given for longest-lived isotope) half-life | 79 Myr (megayears)
Thermodynamic properties
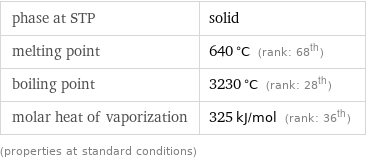
phase at STP | solid melting point | 640 °C (rank: 68th) boiling point | 3230 °C (rank: 28th) molar heat of vaporization | 325 kJ/mol (rank: 36th) (properties at standard conditions)
Material properties
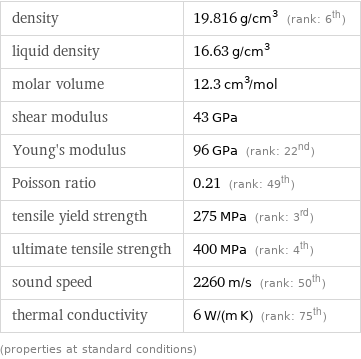
density | 19.816 g/cm^3 (rank: 6th) liquid density | 16.63 g/cm^3 molar volume | 12.3 cm^3/mol shear modulus | 43 GPa Young's modulus | 96 GPa (rank: 22nd) Poisson ratio | 0.21 (rank: 49th) tensile yield strength | 275 MPa (rank: 3rd) ultimate tensile strength | 400 MPa (rank: 4th) sound speed | 2260 m/s (rank: 50th) thermal conductivity | 6 W/(m K) (rank: 75th) (properties at standard conditions)
Electromagnetic properties
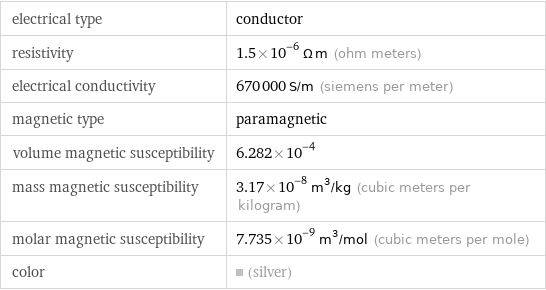
electrical type | conductor resistivity | 1.5×10^-6 Ω m (ohm meters) electrical conductivity | 670000 S/m (siemens per meter) magnetic type | paramagnetic volume magnetic susceptibility | 6.282×10^-4 mass magnetic susceptibility | 3.17×10^-8 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 7.735×10^-9 m^3/mol (cubic meters per mole) color | (silver)
Reactivity
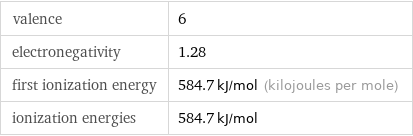
valence | 6 electronegativity | 1.28 first ionization energy | 584.7 kJ/mol (kilojoules per mole) ionization energies | 584.7 kJ/mol
Atomic properties

term symbol | ^7F_0 atomic radius | 175 pm covalent radius | 187 pm (electronic ground state properties)
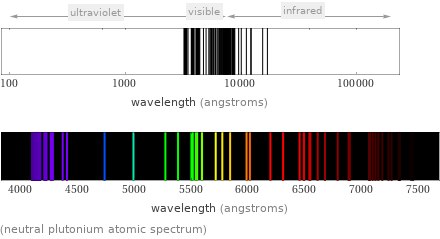
(neutral plutonium atomic spectrum)
Abundances
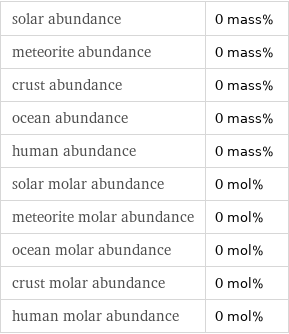
solar abundance | 0 mass% meteorite abundance | 0 mass% crust abundance | 0 mass% ocean abundance | 0 mass% human abundance | 0 mass% solar molar abundance | 0 mol% meteorite molar abundance | 0 mol% ocean molar abundance | 0 mol% crust molar abundance | 0 mol% human molar abundance | 0 mol%
Nuclear properties
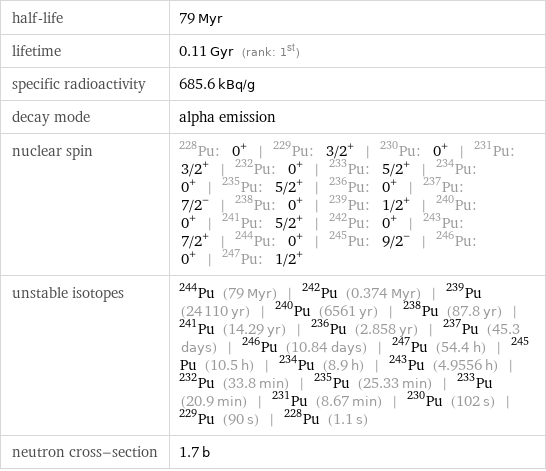
half-life | 79 Myr lifetime | 0.11 Gyr (rank: 1st) specific radioactivity | 685.6 kBq/g decay mode | alpha emission nuclear spin | Pu-228: 0^+ | Pu-229: 3/2^+ | Pu-230: 0^+ | Pu-231: 3/2^+ | Pu-232: 0^+ | Pu-233: 5/2^+ | Pu-234: 0^+ | Pu-235: 5/2^+ | Pu-236: 0^+ | Pu-237: 7/2^- | Pu-238: 0^+ | Pu-239: 1/2^+ | Pu-240: 0^+ | Pu-241: 5/2^+ | Pu-242: 0^+ | Pu-243: 7/2^+ | Pu-244: 0^+ | Pu-245: 9/2^- | Pu-246: 0^+ | Pu-247: 1/2^+ unstable isotopes | Pu-244 (79 Myr) | Pu-242 (0.374 Myr) | Pu-239 (24110 yr) | Pu-240 (6561 yr) | Pu-238 (87.8 yr) | Pu-241 (14.29 yr) | Pu-236 (2.858 yr) | Pu-237 (45.3 days) | Pu-246 (10.84 days) | Pu-247 (54.4 h) | Pu-245 (10.5 h) | Pu-234 (8.9 h) | Pu-243 (4.9556 h) | Pu-232 (33.8 min) | Pu-235 (25.33 min) | Pu-233 (20.9 min) | Pu-231 (8.67 min) | Pu-230 (102 s) | Pu-229 (90 s) | Pu-228 (1.1 s) neutron cross-section | 1.7 b
Identifiers
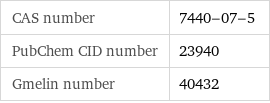
CAS number | 7440-07-5 PubChem CID number | 23940 Gmelin number | 40432