Input interpretation
sulfur (chemical element)
Periodic table location
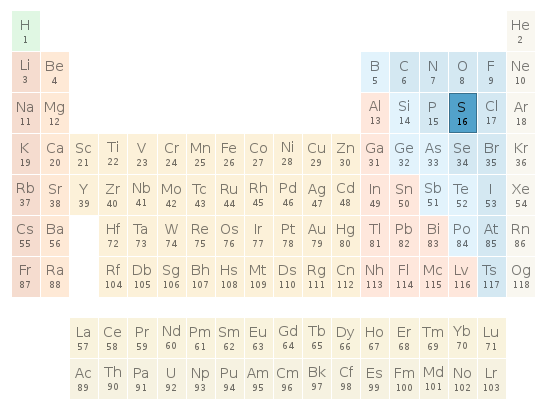
Periodic table location
Image

Image
Basic elemental properties
![atomic symbol | S atomic number | 16 short electronic configuration | [Ne]3s^23p^4 Aufbau diagram | 3p 3s block | p group | 16 period | 3 atomic mass | 32.06 u (unified atomic mass units)](../image_source/a895cb2006638dfa9f653feee8a573d0.png)
atomic symbol | S atomic number | 16 short electronic configuration | [Ne]3s^23p^4 Aufbau diagram | 3p 3s block | p group | 16 period | 3 atomic mass | 32.06 u (unified atomic mass units)
Thermodynamic properties
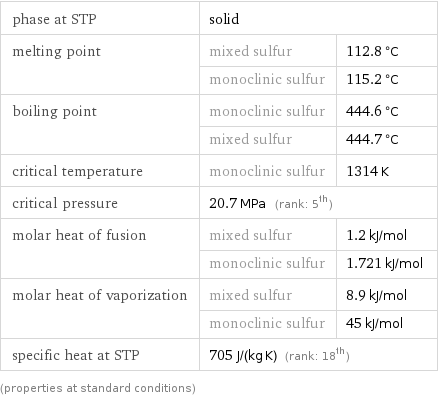
phase at STP | solid | melting point | mixed sulfur | 112.8 °C | monoclinic sulfur | 115.2 °C boiling point | monoclinic sulfur | 444.6 °C | mixed sulfur | 444.7 °C critical temperature | monoclinic sulfur | 1314 K critical pressure | 20.7 MPa (rank: 5th) | molar heat of fusion | mixed sulfur | 1.2 kJ/mol | monoclinic sulfur | 1.721 kJ/mol molar heat of vaporization | mixed sulfur | 8.9 kJ/mol | monoclinic sulfur | 45 kJ/mol specific heat at STP | 705 J/(kg K) (rank: 18th) | (properties at standard conditions)
Material properties
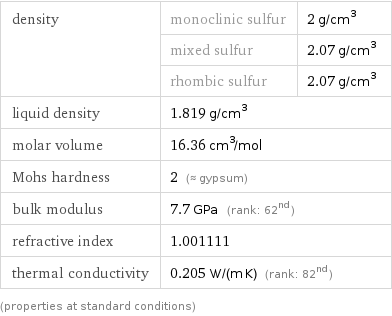
density | monoclinic sulfur | 2 g/cm^3 | mixed sulfur | 2.07 g/cm^3 | rhombic sulfur | 2.07 g/cm^3 liquid density | 1.819 g/cm^3 | molar volume | 16.36 cm^3/mol | Mohs hardness | 2 (≈ gypsum) | bulk modulus | 7.7 GPa (rank: 62nd) | refractive index | 1.001111 | thermal conductivity | 0.205 W/(m K) (rank: 82nd) | (properties at standard conditions)
Electromagnetic properties
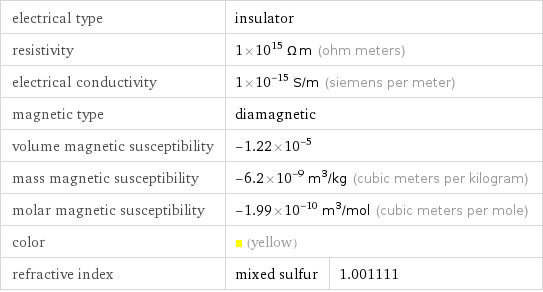
electrical type | insulator | resistivity | 1×10^15 Ω m (ohm meters) | electrical conductivity | 1×10^-15 S/m (siemens per meter) | magnetic type | diamagnetic | volume magnetic susceptibility | -1.22×10^-5 | mass magnetic susceptibility | -6.2×10^-9 m^3/kg (cubic meters per kilogram) | molar magnetic susceptibility | -1.99×10^-10 m^3/mol (cubic meters per mole) | color | (yellow) | refractive index | mixed sulfur | 1.001111
Reactivity
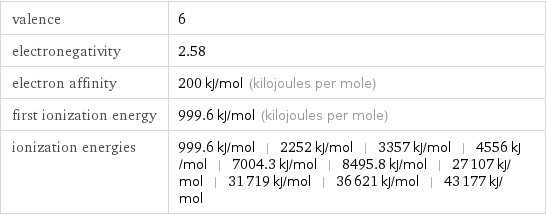
valence | 6 electronegativity | 2.58 electron affinity | 200 kJ/mol (kilojoules per mole) first ionization energy | 999.6 kJ/mol (kilojoules per mole) ionization energies | 999.6 kJ/mol | 2252 kJ/mol | 3357 kJ/mol | 4556 kJ/mol | 7004.3 kJ/mol | 8495.8 kJ/mol | 27107 kJ/mol | 31719 kJ/mol | 36621 kJ/mol | 43177 kJ/mol
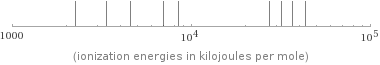
Reactivity