Input interpretation
p block ions
Members

helium cation | nitride anion | oxide anion | fluoride anion | aluminum cation | phosphide anion | sulfide anion | chloride anion | gallium(III) cation | arsenic(III) cation | arsenic(V) cation | arsenide anion | selenide anion | bromide anion | indium(III) cation | tin(II) cation | tin(IV) cation | antimony(III) cation | antimony(V) cation | iodide anion (total: 20)
Ionic radii
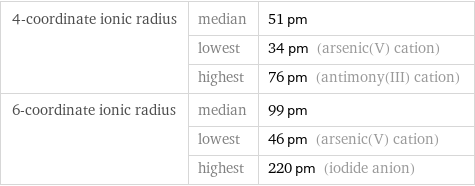
4-coordinate ionic radius | median | 51 pm | lowest | 34 pm (arsenic(V) cation) | highest | 76 pm (antimony(III) cation) 6-coordinate ionic radius | median | 99 pm | lowest | 46 pm (arsenic(V) cation) | highest | 220 pm (iodide anion)
Units
