Input interpretation

strong electrolytes | molar heat of fusion
Summary
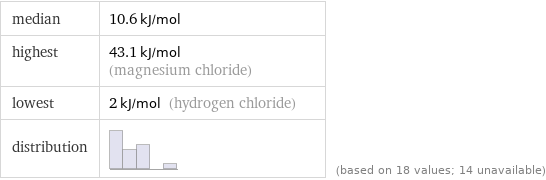
median | 10.6 kJ/mol highest | 43.1 kJ/mol (magnesium chloride) lowest | 2 kJ/mol (hydrogen chloride) distribution | | (based on 18 values; 14 unavailable)
Entities with missing values
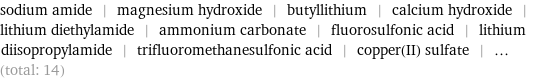
sodium amide | magnesium hydroxide | butyllithium | calcium hydroxide | lithium diethylamide | ammonium carbonate | fluorosulfonic acid | lithium diisopropylamide | trifluoromethanesulfonic acid | copper(II) sulfate | ... (total: 14)
Distribution plots
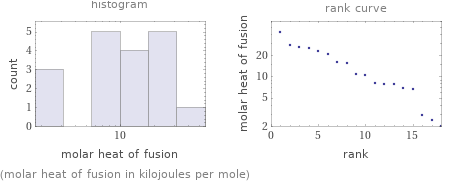
(molar heat of fusion in kilojoules per mole)
Molar heat of fusion rankings
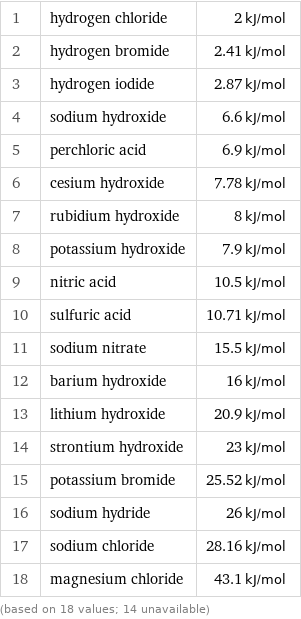
1 | hydrogen chloride | 2 kJ/mol 2 | hydrogen bromide | 2.41 kJ/mol 3 | hydrogen iodide | 2.87 kJ/mol 4 | sodium hydroxide | 6.6 kJ/mol 5 | perchloric acid | 6.9 kJ/mol 6 | cesium hydroxide | 7.78 kJ/mol 7 | rubidium hydroxide | 8 kJ/mol 8 | potassium hydroxide | 7.9 kJ/mol 9 | nitric acid | 10.5 kJ/mol 10 | sulfuric acid | 10.71 kJ/mol 11 | sodium nitrate | 15.5 kJ/mol 12 | barium hydroxide | 16 kJ/mol 13 | lithium hydroxide | 20.9 kJ/mol 14 | strontium hydroxide | 23 kJ/mol 15 | potassium bromide | 25.52 kJ/mol 16 | sodium hydride | 26 kJ/mol 17 | sodium chloride | 28.16 kJ/mol 18 | magnesium chloride | 43.1 kJ/mol (based on 18 values; 14 unavailable)
Unit conversions for median molar heat of fusion 10.6 kJ/mol
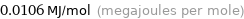
0.0106 MJ/mol (megajoules per mole)
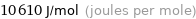
10610 J/mol (joules per mole)

2.53 kcal/mol (thermochemical kilocalories per mole)
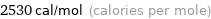
2530 cal/mol (calories per mole)
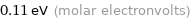
0.11 eV (molar electronvolts)