Input interpretation

period 2 elements | molar heat of vaporization
Summary
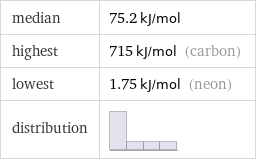
median | 75.2 kJ/mol highest | 715 kJ/mol (carbon) lowest | 1.75 kJ/mol (neon) distribution |
Units

Ranked values
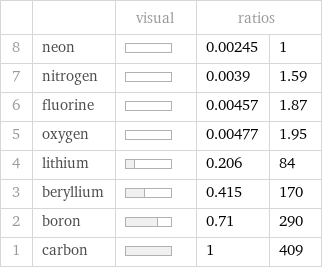
| | visual | ratios | 8 | neon | | 0.00245 | 1 7 | nitrogen | | 0.0039 | 1.59 6 | fluorine | | 0.00457 | 1.87 5 | oxygen | | 0.00477 | 1.95 4 | lithium | | 0.206 | 84 3 | beryllium | | 0.415 | 170 2 | boron | | 0.71 | 290 1 | carbon | | 1 | 409
Molar heat of vaporization rankings
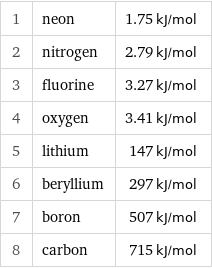
1 | neon | 1.75 kJ/mol 2 | nitrogen | 2.79 kJ/mol 3 | fluorine | 3.27 kJ/mol 4 | oxygen | 3.41 kJ/mol 5 | lithium | 147 kJ/mol 6 | beryllium | 297 kJ/mol 7 | boron | 507 kJ/mol 8 | carbon | 715 kJ/mol
Unit conversions for median molar heat of vaporization 75.2 kJ/mol
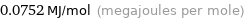
0.0752 MJ/mol (megajoules per mole)
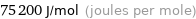
75200 J/mol (joules per mole)
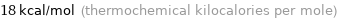
18 kcal/mol (thermochemical kilocalories per mole)
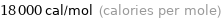
18000 cal/mol (calories per mole)

0.779 eV (molar electronvolts)