Input interpretation

sulfuryl chloride fluoride
Chemical names and formulas
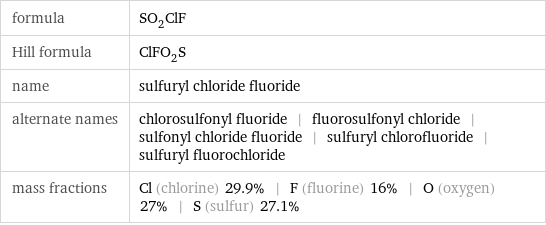
formula | SO_2ClF Hill formula | ClFO_2S name | sulfuryl chloride fluoride alternate names | chlorosulfonyl fluoride | fluorosulfonyl chloride | sulfonyl chloride fluoride | sulfuryl chlorofluoride | sulfuryl fluorochloride mass fractions | Cl (chlorine) 29.9% | F (fluorine) 16% | O (oxygen) 27% | S (sulfur) 27.1%
Lewis structure
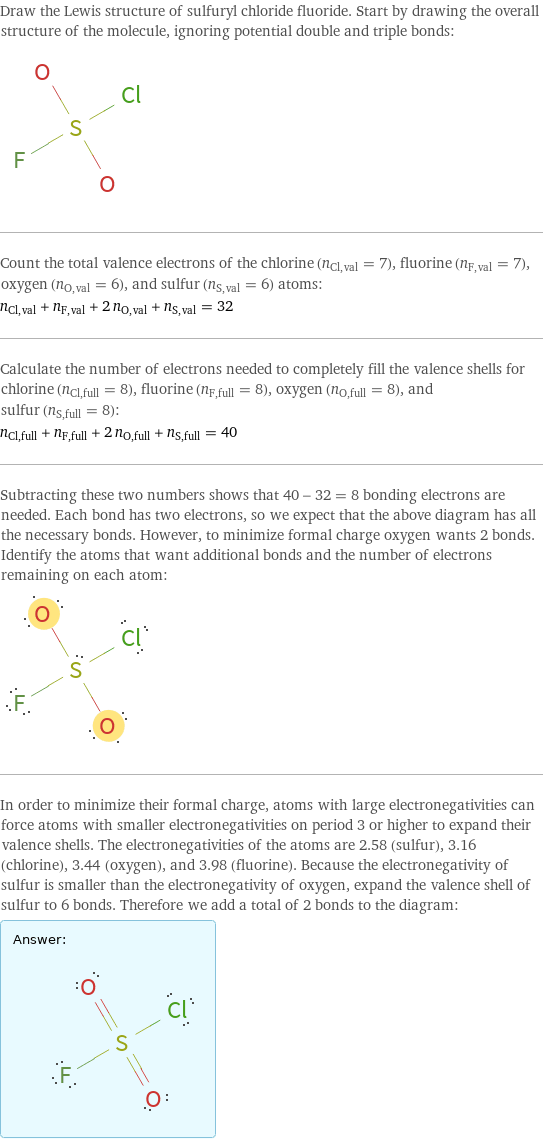
Draw the Lewis structure of sulfuryl chloride fluoride. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the chlorine (n_Cl, val = 7), fluorine (n_F, val = 7), oxygen (n_O, val = 6), and sulfur (n_S, val = 6) atoms: n_Cl, val + n_F, val + 2 n_O, val + n_S, val = 32 Calculate the number of electrons needed to completely fill the valence shells for chlorine (n_Cl, full = 8), fluorine (n_F, full = 8), oxygen (n_O, full = 8), and sulfur (n_S, full = 8): n_Cl, full + n_F, full + 2 n_O, full + n_S, full = 40 Subtracting these two numbers shows that 40 - 32 = 8 bonding electrons are needed. Each bond has two electrons, so we expect that the above diagram has all the necessary bonds. However, to minimize formal charge oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: In order to minimize their formal charge, atoms with large electronegativities can force atoms with smaller electronegativities on period 3 or higher to expand their valence shells. The electronegativities of the atoms are 2.58 (sulfur), 3.16 (chlorine), 3.44 (oxygen), and 3.98 (fluorine). Because the electronegativity of sulfur is smaller than the electronegativity of oxygen, expand the valence shell of sulfur to 6 bonds. Therefore we add a total of 2 bonds to the diagram: Answer: | |
3D structure

3D structure
Basic properties

molar mass | 118.5 g/mol phase | gas (at STP) melting point | -125 °C boiling point | 7 °C density | 1.623 g/cm^3 (at 25 °C) solubility in water | soluble
Units

Gas properties (at STP)
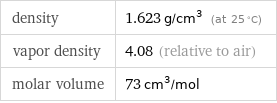
density | 1.623 g/cm^3 (at 25 °C) vapor density | 4.08 (relative to air) molar volume | 73 cm^3/mol
Units

Thermodynamic properties

molar heat of vaporization | 26.5 kJ/mol specific heat of vaporization | 0.224 kJ/g (at STP)
Chemical identifiers
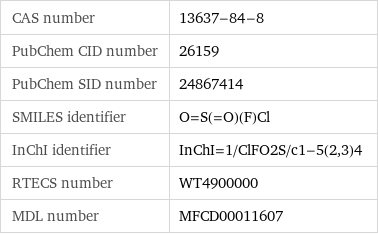
CAS number | 13637-84-8 PubChem CID number | 26159 PubChem SID number | 24867414 SMILES identifier | O=S(=O)(F)Cl InChI identifier | InChI=1/ClFO2S/c1-5(2, 3)4 RTECS number | WT4900000 MDL number | MFCD00011607
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 1 NFPA reactivity rating | 2
Safety properties

flash point | 109 °C

DOT hazard class | 8 DOT numbers | 1760
Toxicity properties

RTECS classes | other