Input interpretation

0.0600 M silver nitrate from 100 mg (milligrams) | preparation
Results

dissolve 100 mg of silver nitrate to a final volume of 9.81 mL
Possible intermediate steps
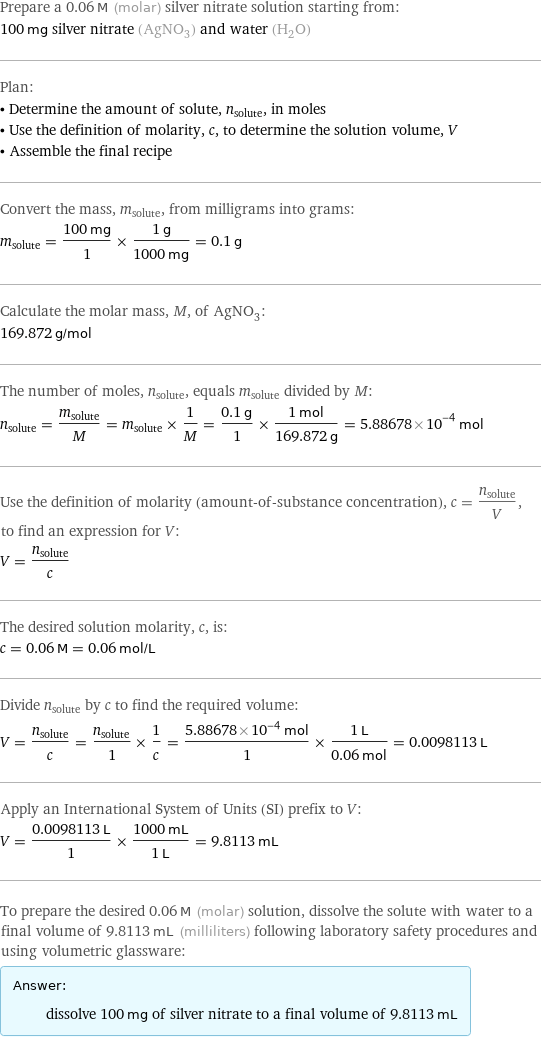
Prepare a 0.06 M (molar) silver nitrate solution starting from: 100 mg silver nitrate (AgNO_3) and water (H_2O) Plan: • Determine the amount of solute, n_solute, in moles • Use the definition of molarity, c, to determine the solution volume, V • Assemble the final recipe Convert the mass, m_solute, from milligrams into grams: m_solute = (100 mg)/1 × (1 g)/(1000 mg) = 0.1 g Calculate the molar mass, M, of AgNO_3: 169.872 g/mol The number of moles, n_solute, equals m_solute divided by M: n_solute = m_solute/M = m_solute × 1/M = (0.1 g)/1 × (1 mol)/(169.872 g) = 5.88678×10^-4 mol Use the definition of molarity (amount-of-substance concentration), c = n_solute/V, to find an expression for V: V = n_solute/c The desired solution molarity, c, is: c = 0.06 M = 0.06 mol/L Divide n_solute by c to find the required volume: V = n_solute/c = n_solute/1 × 1/c = (5.88678×10^-4 mol)/1 × (1 L)/(0.06 mol) = 0.0098113 L Apply an International System of Units (SI) prefix to V: V = (0.0098113 L)/1 × (1000 mL)/(1 L) = 9.8113 mL To prepare the desired 0.06 M (molar) solution, dissolve the solute with water to a final volume of 9.8113 mL (milliliters) following laboratory safety procedures and using volumetric glassware: Answer: | | dissolve 100 mg of silver nitrate to a final volume of 9.8113 mL