Input interpretation
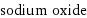
sodium oxide
Chemical names and formulas

formula | Na_2O name | sodium oxide IUPAC name | disodium oxygen(-2) anion alternate names | disodium oxygen(-2) anion mass fractions | Na (sodium) 74.2% | O (oxygen) 25.8%
Structure diagram
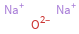
Structure diagram
Basic properties
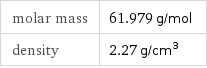
molar mass | 61.979 g/mol density | 2.27 g/cm^3
Units

Thermodynamic properties

specific heat capacity c_p | solid | 1.115 J/(g K) molar heat capacity c_p | solid | 69.1 J/(mol K) specific free energy of formation Δ_fG° | solid | -6.059 kJ/g molar free energy of formation Δ_fG° | solid | -375.5 kJ/mol specific heat of formation Δ_fH° | solid | -6.683 kJ/g molar heat of formation Δ_fH° | solid | -414.2 kJ/mol specific entropy S° | solid | 1.178 J/(g K) molar entropy S° | solid | 73 J/(mol K) molar heat of fusion | 47.7 kJ/mol | specific heat of fusion | 0.77 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1313-59-3 PubChem CID number | 73971 SMILES identifier | [O-2].[Na+].[Na+] InChI identifier | InChI=1/2Na.O/q2*+1;-2 MDL number | MFCD00046201](../image_source/a2768f98064c6a7cafba47d7034e6a09.png)
CAS number | 1313-59-3 PubChem CID number | 73971 SMILES identifier | [O-2].[Na+].[Na+] InChI identifier | InChI=1/2Na.O/q2*+1;-2 MDL number | MFCD00046201
Ion equivalents

Na^+ (sodium cation) | 2 O^(2-) (oxide anion) | 1