Input interpretation
L-cystine
Chemical names and formulas
![formula | C_6H_12N_2O_4S_2 name | L-cystine IUPAC name | (2S)-2-azaniumyl-3-[(2S)-2-azaniumyl-2-carboxylato-ethyl]disulfanyl-propanoate alternate names | 3, 3'-dithiobis(2-aminopropionic acid) | cystin | cystine mass fractions | C (carbon) 30% | H (hydrogen) 5.03% | N (nitrogen) 11.7% | O (oxygen) 26.6% | S (sulfur) 26.7%](../image_source/f0970dfe6e92f025e63f9f3be4cf5fd6.png)
formula | C_6H_12N_2O_4S_2 name | L-cystine IUPAC name | (2S)-2-azaniumyl-3-[(2S)-2-azaniumyl-2-carboxylato-ethyl]disulfanyl-propanoate alternate names | 3, 3'-dithiobis(2-aminopropionic acid) | cystin | cystine mass fractions | C (carbon) 30% | H (hydrogen) 5.03% | N (nitrogen) 11.7% | O (oxygen) 26.6% | S (sulfur) 26.7%
Lewis structure
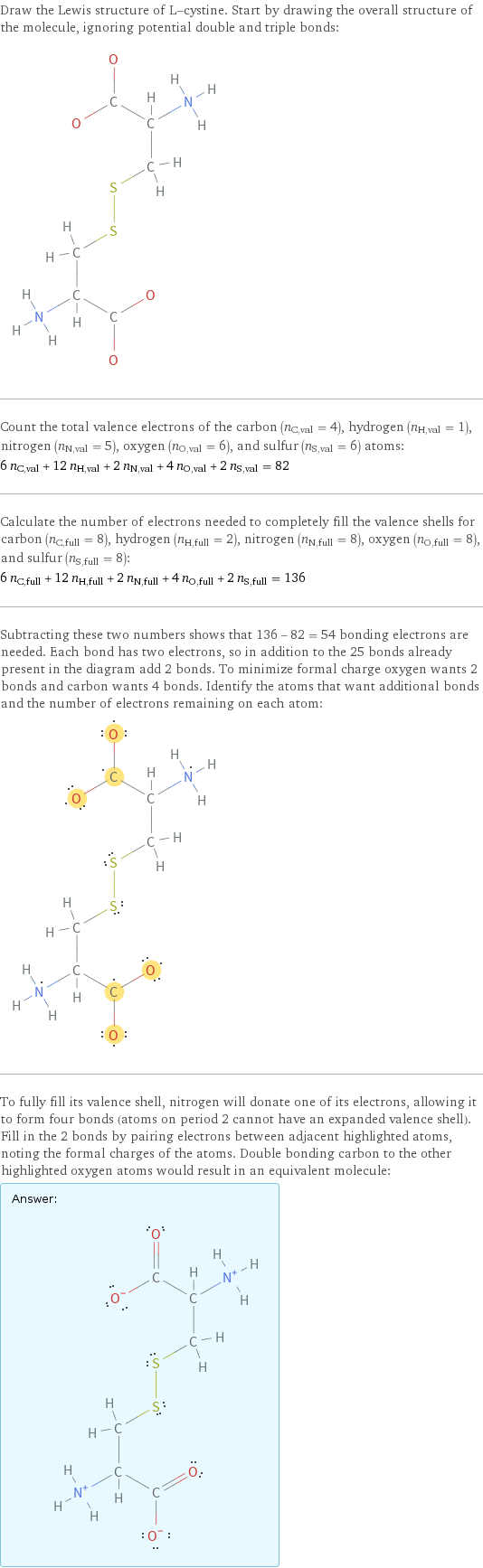
Draw the Lewis structure of L-cystine. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), oxygen (n_O, val = 6), and sulfur (n_S, val = 6) atoms: 6 n_C, val + 12 n_H, val + 2 n_N, val + 4 n_O, val + 2 n_S, val = 82 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), oxygen (n_O, full = 8), and sulfur (n_S, full = 8): 6 n_C, full + 12 n_H, full + 2 n_N, full + 4 n_O, full + 2 n_S, full = 136 Subtracting these two numbers shows that 136 - 82 = 54 bonding electrons are needed. Each bond has two electrons, so in addition to the 25 bonds already present in the diagram add 2 bonds. To minimize formal charge oxygen wants 2 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: To fully fill its valence shell, nitrogen will donate one of its electrons, allowing it to form four bonds (atoms on period 2 cannot have an expanded valence shell). Fill in the 2 bonds by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms. Double bonding carbon to the other highlighted oxygen atoms would result in an equivalent molecule: Answer: | |
3D structure
3D structure
Basic properties
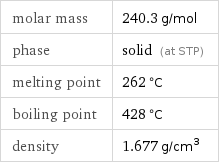
molar mass | 240.3 g/mol phase | solid (at STP) melting point | 262 °C boiling point | 428 °C density | 1.677 g/cm^3
Units

Hydrophobicity and permeability properties

experimental LogP hydrophobicity | -5.5 predicted LogP hydrophobicity | -3.16 predicted LogS | -1.16
Basic drug properties

approval status | approved | nutraceutical | small molecule drug categories | dietary supplement | micronutrient | non-essential amino acid
Solid properties (at STP)

density | 1.677 g/cm^3
Units

Thermodynamic properties

specific heat of formation Δ_fH° | solid | -4.298 kJ/g molar heat of formation Δ_fH° | solid | -1033 kJ/mol (at STP)
Chemical identifiers
![CAS number | 56-89-3 Beilstein number | 1728094 PubChem CID number | 6992103 PubChem SID number | 3774 SMILES identifier | C(C(C(=O)[O-])[NH3+])SSCC(C(=O)[O-])[NH3+] InChI identifier | InChI=1/C6H12N2O4S2/c7-3(5(9)10)1-13-14-2-4(8)6(11)12/h3-4H, 1-2, 7-8H2, (H, 9, 10)(H, 11, 12)/t3-, 4-/m0/s1/f/h7-8H InChI key | LEVWYRKDKASIDU-JYGOYGMTDR EU number | 200-296-3 RTECS number | HA2690000 NSC number | 13203](../image_source/8ec97a111ddb45ca6c5369b16a53d392.png)
CAS number | 56-89-3 Beilstein number | 1728094 PubChem CID number | 6992103 PubChem SID number | 3774 SMILES identifier | C(C(C(=O)[O-])[NH3+])SSCC(C(=O)[O-])[NH3+] InChI identifier | InChI=1/C6H12N2O4S2/c7-3(5(9)10)1-13-14-2-4(8)6(11)12/h3-4H, 1-2, 7-8H2, (H, 9, 10)(H, 11, 12)/t3-, 4-/m0/s1/f/h7-8H InChI key | LEVWYRKDKASIDU-JYGOYGMTDR EU number | 200-296-3 RTECS number | HA2690000 NSC number | 13203
Toxicity properties

RTECS classes | drug