Input interpretation
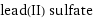
lead(II) sulfate
Chemical names and formulas
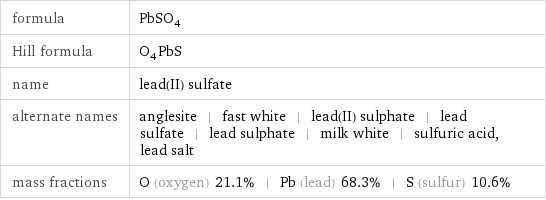
formula | PbSO_4 Hill formula | O_4PbS name | lead(II) sulfate alternate names | anglesite | fast white | lead(II) sulphate | lead sulfate | lead sulphate | milk white | sulfuric acid, lead salt mass fractions | O (oxygen) 21.1% | Pb (lead) 68.3% | S (sulfur) 10.6%
Structure diagram
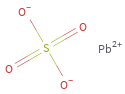
Structure diagram
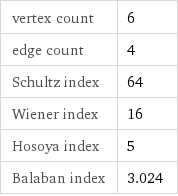
vertex count | 6 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
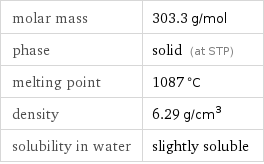
molar mass | 303.3 g/mol phase | solid (at STP) melting point | 1087 °C density | 6.29 g/cm^3 solubility in water | slightly soluble
Units

Solid properties (at STP)

density | 6.29 g/cm^3 refractive index | 1.8848
Units

Thermodynamic properties
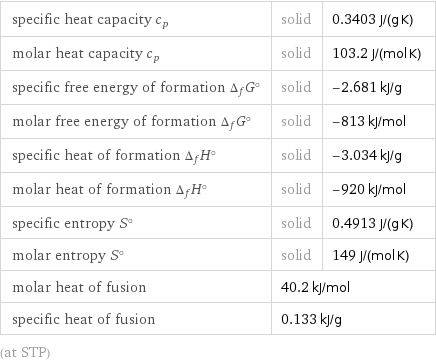
specific heat capacity c_p | solid | 0.3403 J/(g K) molar heat capacity c_p | solid | 103.2 J/(mol K) specific free energy of formation Δ_fG° | solid | -2.681 kJ/g molar free energy of formation Δ_fG° | solid | -813 kJ/mol specific heat of formation Δ_fH° | solid | -3.034 kJ/g molar heat of formation Δ_fH° | solid | -920 kJ/mol specific entropy S° | solid | 0.4913 J/(g K) molar entropy S° | solid | 149 J/(mol K) molar heat of fusion | 40.2 kJ/mol | specific heat of fusion | 0.133 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7446-14-2 SMILES identifier | O=S(=O)([O-])[O-].[Pb+2] EU number | 239-831-0 Gmelin number | 18674 RTECS number | OG4375000 MDL number | MFCD00011166](../image_source/7c22db3de2a28717ff02d459c77e0444.png)
CAS number | 7446-14-2 SMILES identifier | O=S(=O)([O-])[O-].[Pb+2] EU number | 239-831-0 Gmelin number | 18674 RTECS number | OG4375000 MDL number | MFCD00011166
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | mutagen