Input interpretation

L-cystine | orbital hybridization
Result
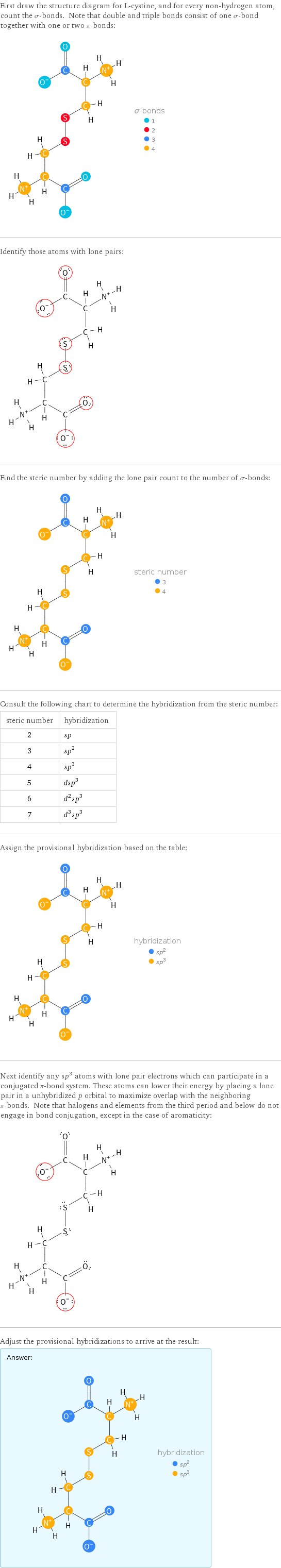
First draw the structure diagram for L-cystine, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: Identify those atoms with lone pairs: Find the steric number by adding the lone pair count to the number of σ-bonds: Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Assign the provisional hybridization based on the table: Next identify any sp^3 atoms with lone pair electrons which can participate in a conjugated π-bond system. These atoms can lower their energy by placing a lone pair in a unhybridized p orbital to maximize overlap with the neighboring π-bonds. Note that halogens and elements from the third period and below do not engage in bond conjugation, except in the case of aromaticity: Adjust the provisional hybridizations to arrive at the result: Answer: | |
Chemical names and formulas
![formula | C_6H_12N_2O_4S_2 name | L-cystine IUPAC name | (2S)-2-azaniumyl-3-[(2S)-2-azaniumyl-2-carboxylato-ethyl]disulfanyl-propanoate alternate names | 3, 3'-dithiobis(2-aminopropionic acid) | cystin | cystine mass fractions | C (carbon) 30% | H (hydrogen) 5.03% | N (nitrogen) 11.7% | O (oxygen) 26.6% | S (sulfur) 26.7%](../image_source/4eb3a94af28e9bc4999f72323ee251de.png)
formula | C_6H_12N_2O_4S_2 name | L-cystine IUPAC name | (2S)-2-azaniumyl-3-[(2S)-2-azaniumyl-2-carboxylato-ethyl]disulfanyl-propanoate alternate names | 3, 3'-dithiobis(2-aminopropionic acid) | cystin | cystine mass fractions | C (carbon) 30% | H (hydrogen) 5.03% | N (nitrogen) 11.7% | O (oxygen) 26.6% | S (sulfur) 26.7%