Input interpretation

group 11 elements | atomic diameter
Results
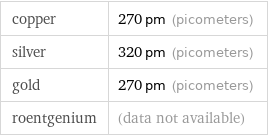
copper | 270 pm (picometers) silver | 320 pm (picometers) gold | 270 pm (picometers) roentgenium | (data not available)

group 11 elements | atomic diameter | 270 pm (picometers) (median) (based on 3 values; 1 unavailable)
Ranked values

| | visual | ratios | 3 | copper | | 0.84 | 1 2 | gold | | 0.84 | 1 1 | silver | | 1 | 1.19
Plot
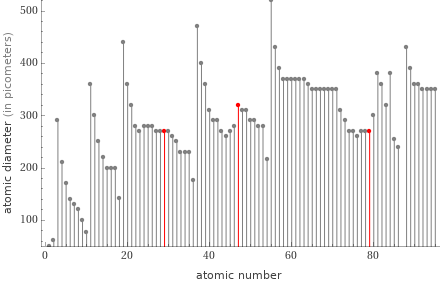
Plot
Periodic table location
Periodic table location
Atomic radii properties
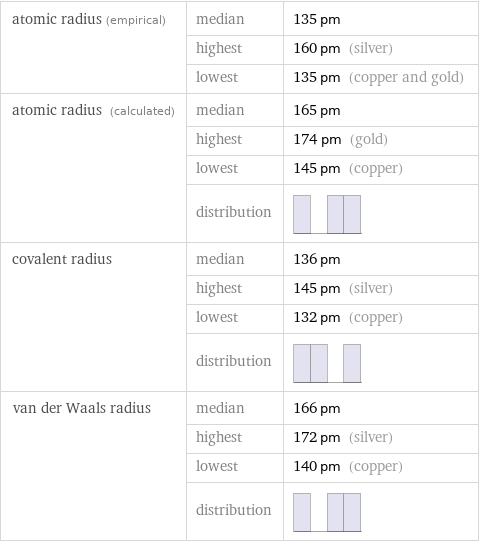
atomic radius (empirical) | median | 135 pm | highest | 160 pm (silver) | lowest | 135 pm (copper and gold) atomic radius (calculated) | median | 165 pm | highest | 174 pm (gold) | lowest | 145 pm (copper) | distribution | covalent radius | median | 136 pm | highest | 145 pm (silver) | lowest | 132 pm (copper) | distribution | van der Waals radius | median | 166 pm | highest | 172 pm (silver) | lowest | 140 pm (copper) | distribution |
Units

Unit conversions for median atomic diameter 270 pm
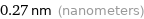
0.27 nm (nanometers)
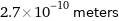
2.7×10^-10 meters
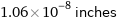
1.06×10^-8 inches
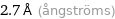
2.7 Å (ångströms)
Comparisons for median atomic diameter 270 pm
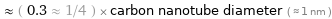
≈ ( 0.3 ≈ 1/4 ) × carbon nanotube diameter ( ≈ 1 nm )
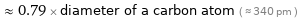
≈ 0.79 × diameter of a carbon atom ( ≈ 340 pm )
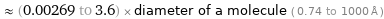
≈ (0.00269 to 3.6) × diameter of a molecule ( 0.74 to 1000 Å )