Input interpretation

aluminum | molecular mass
Result
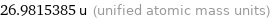
26.9815385 u (unified atomic mass units)
Unit conversions
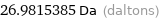
26.9815385 Da (daltons)
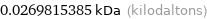
0.0269815385 kDa (kilodaltons)
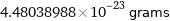
4.48038988×10^-23 grams
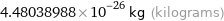
4.48038988×10^-26 kg (kilograms)

26.9827 chemical atomic mass units (unit officially deprecated)
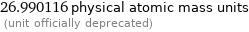
26.990116 physical atomic mass units (unit officially deprecated)
Comparisons as mass of molecule
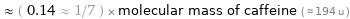
≈ ( 0.14 ≈ 1/7 ) × molecular mass of caffeine ( ≈ 194 u )
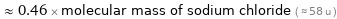
≈ 0.46 × molecular mass of sodium chloride ( ≈ 58 u )
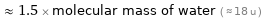
≈ 1.5 × molecular mass of water ( ≈ 18 u )
Corresponding quantities
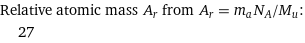
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 27
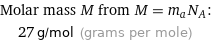
Molar mass M from M = m_aN_A: | 27 g/mol (grams per mole)
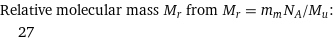
Relative molecular mass M_r from M_r = m_mN_A/M_u: | 27
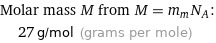
Molar mass M from M = m_mN_A: | 27 g/mol (grams per mole)