Input interpretation
lithium nitrate | lithium fluoride
Chemical names and formulas
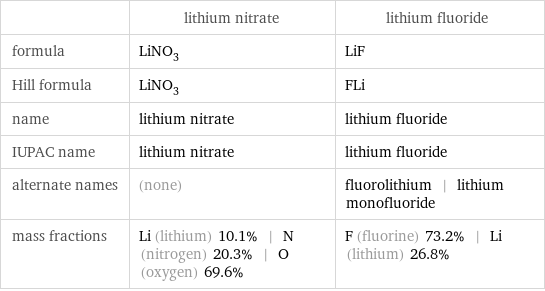
| lithium nitrate | lithium fluoride formula | LiNO_3 | LiF Hill formula | LiNO_3 | FLi name | lithium nitrate | lithium fluoride IUPAC name | lithium nitrate | lithium fluoride alternate names | (none) | fluorolithium | lithium monofluoride mass fractions | Li (lithium) 10.1% | N (nitrogen) 20.3% | O (oxygen) 69.6% | F (fluorine) 73.2% | Li (lithium) 26.8%
Structure diagrams
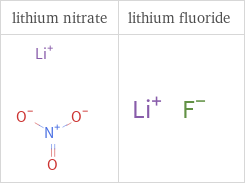
Structure diagrams
Basic properties
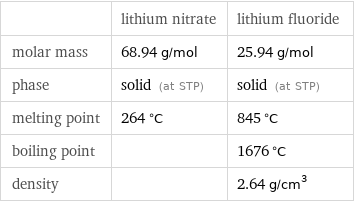
| lithium nitrate | lithium fluoride molar mass | 68.94 g/mol | 25.94 g/mol phase | solid (at STP) | solid (at STP) melting point | 264 °C | 845 °C boiling point | | 1676 °C density | | 2.64 g/cm^3
Units

Thermodynamic properties
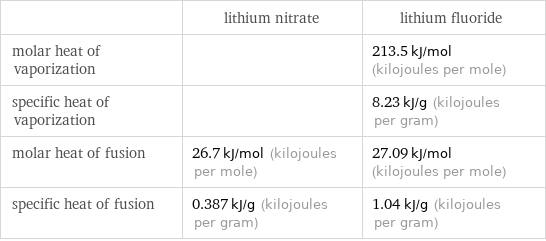
| lithium nitrate | lithium fluoride molar heat of vaporization | | 213.5 kJ/mol (kilojoules per mole) specific heat of vaporization | | 8.23 kJ/g (kilojoules per gram) molar heat of fusion | 26.7 kJ/mol (kilojoules per mole) | 27.09 kJ/mol (kilojoules per mole) specific heat of fusion | 0.387 kJ/g (kilojoules per gram) | 1.04 kJ/g (kilojoules per gram)
Solid properties (at STP)
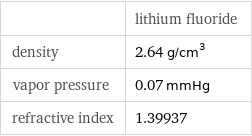
| lithium fluoride density | 2.64 g/cm^3 vapor pressure | 0.07 mmHg refractive index | 1.39937
Units

Chemical identifiers
![| lithium nitrate | lithium fluoride CAS number | 7790-69-4 | 7789-24-4 PubChem CID number | 10129889 | 224478 PubChem SID number | 24853743 | 24852188 SMILES identifier | [Li+].[N+](=O)([O-])[O-] | [Li+].[F-] InChI identifier | InChI=1/Li.NO3/c;2-1(3)4/q+1;-1 | InChI=1/FH.Li/h1H;/q;+1/p-1/fF.Li/h1h;/q-1;m RTECS number | | OJ6125000 MDL number | MFCD00011094 | MFCD00011090](../image_source/228aa2d1dcc0633fdc56e6fee848d670.png)
| lithium nitrate | lithium fluoride CAS number | 7790-69-4 | 7789-24-4 PubChem CID number | 10129889 | 224478 PubChem SID number | 24853743 | 24852188 SMILES identifier | [Li+].[N+](=O)([O-])[O-] | [Li+].[F-] InChI identifier | InChI=1/Li.NO3/c;2-1(3)4/q+1;-1 | InChI=1/FH.Li/h1H;/q;+1/p-1/fF.Li/h1h;/q-1;m RTECS number | | OJ6125000 MDL number | MFCD00011094 | MFCD00011090
NFPA label
NFPA label
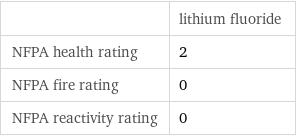
| lithium fluoride NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0