Input interpretation

gaseous elements (at STP) | atomic radius
Summary
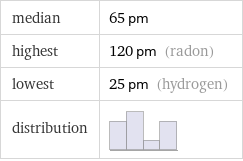
median | 65 pm highest | 120 pm (radon) lowest | 25 pm (hydrogen) distribution |
Units

Plot
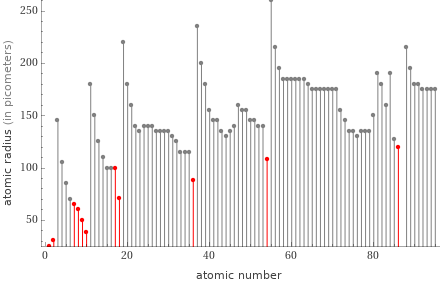
Plot
Periodic table location
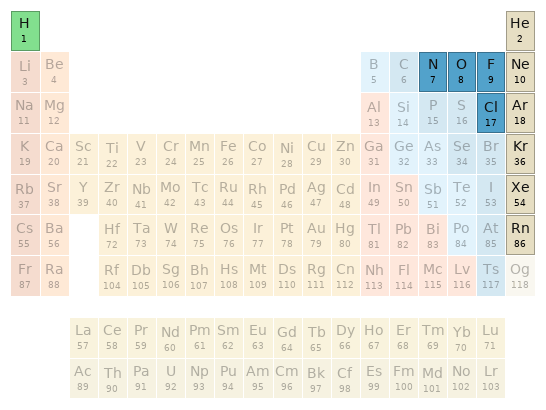
Periodic table location
Atomic radii properties
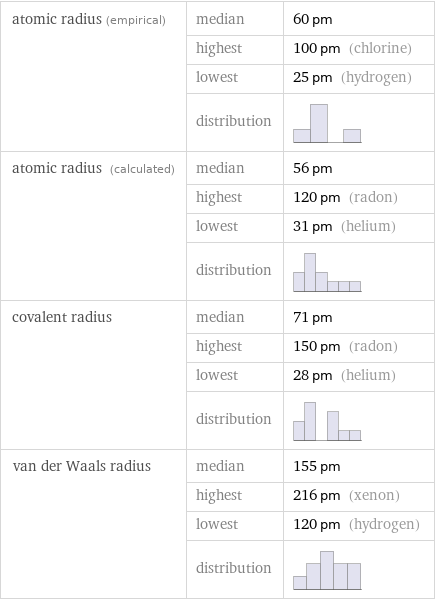
atomic radius (empirical) | median | 60 pm | highest | 100 pm (chlorine) | lowest | 25 pm (hydrogen) | distribution | atomic radius (calculated) | median | 56 pm | highest | 120 pm (radon) | lowest | 31 pm (helium) | distribution | covalent radius | median | 71 pm | highest | 150 pm (radon) | lowest | 28 pm (helium) | distribution | van der Waals radius | median | 155 pm | highest | 216 pm (xenon) | lowest | 120 pm (hydrogen) | distribution |
Units

Distribution plots
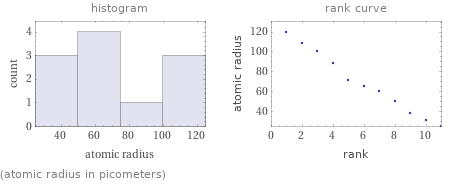
(atomic radius in picometers)
Atomic radius rankings
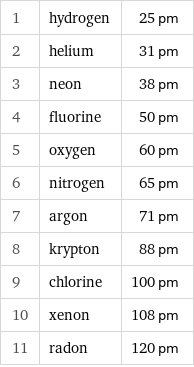
1 | hydrogen | 25 pm 2 | helium | 31 pm 3 | neon | 38 pm 4 | fluorine | 50 pm 5 | oxygen | 60 pm 6 | nitrogen | 65 pm 7 | argon | 71 pm 8 | krypton | 88 pm 9 | chlorine | 100 pm 10 | xenon | 108 pm 11 | radon | 120 pm
Unit conversions for median atomic radius 65 pm
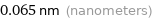
0.065 nm (nanometers)
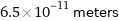
6.5×10^-11 meters
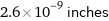
2.6×10^-9 inches
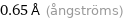
0.65 Å (ångströms)
Comparisons for median atomic radius 65 pm
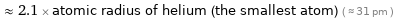
≈ 2.1 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
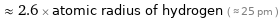
≈ 2.6 × atomic radius of hydrogen ( ≈ 25 pm )