Input interpretation
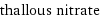
thallous nitrate
Chemical names and formulas
formula | TlNO_3 Hill formula | NO_3Tl name | thallous nitrate IUPAC name | thallium(+1) cation nitrate alternate names | thallium(+1) cation nitrate | thallium(1+) nitrate | thallium(I) nitrate | thallium mononitrate | thallium nitrate mass fractions | N (nitrogen) 5.26% | O (oxygen) 18% | Tl (thallium) 76.7%
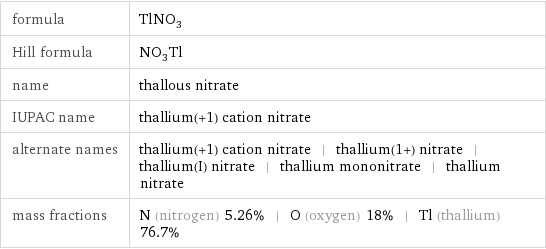
Structure diagram
Structure diagram
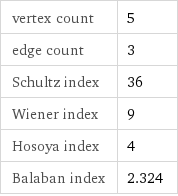
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
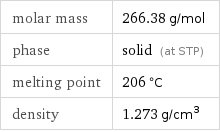
molar mass | 266.38 g/mol phase | solid (at STP) melting point | 206 °C density | 1.273 g/cm^3
Units

Solid properties (at STP)
density | 1.273 g/cm^3
Units

Thermodynamic properties
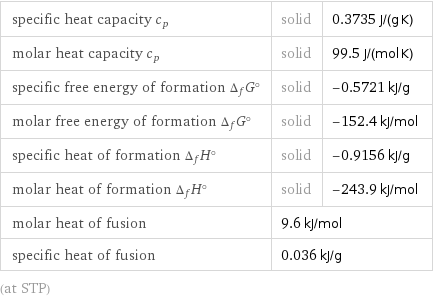
specific heat capacity c_p | solid | 0.3735 J/(g K) molar heat capacity c_p | solid | 99.5 J/(mol K) specific free energy of formation Δ_fG° | solid | -0.5721 kJ/g molar free energy of formation Δ_fG° | solid | -152.4 kJ/mol specific heat of formation Δ_fH° | solid | -0.9156 kJ/g molar heat of formation Δ_fH° | solid | -243.9 kJ/mol molar heat of fusion | 9.6 kJ/mol | specific heat of fusion | 0.036 kJ/g | (at STP)
Chemical identifiers
([O-])[O-].[Tl+] InChI identifier | InChI=1/NO3.Tl/c2-1(3)4;/q-1;+1 RTECS number | XG5950000 MDL number | MFCD00011280](../image_source/9bcf3dfbb5928caed681f13123d20923.png)
CAS number | 10102-45-1 PubChem CID number | 24937 PubChem SID number | 24852281 SMILES identifier | [N+](=O)([O-])[O-].[Tl+] InChI identifier | InChI=1/NO3.Tl/c2-1(3)4;/q-1;+1 RTECS number | XG5950000 MDL number | MFCD00011280
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 1 NFPA reactivity rating | 0
Safety properties

flash point | 450 °C
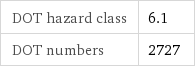
DOT hazard class | 6.1 DOT numbers | 2727
Toxicity properties
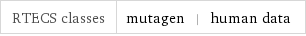
RTECS classes | mutagen | human data
Ion equivalents

Tl^+ (thallium(I) cation) | 1 (NO_3)^- (nitrate anion) | 1