Input interpretation

benzene | aromatic atom count
Result

6 atoms
Aromatic atoms in place
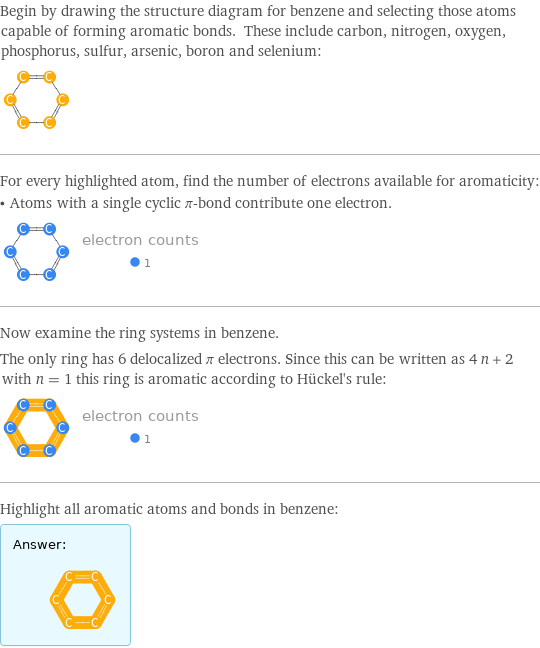
Begin by drawing the structure diagram for benzene and selecting those atoms capable of forming aromatic bonds. These include carbon, nitrogen, oxygen, phosphorus, sulfur, arsenic, boron and selenium: For every highlighted atom, find the number of electrons available for aromaticity: • Atoms with a single cyclic π-bond contribute one electron. Now examine the ring systems in benzene. The only ring has 6 delocalized π electrons. Since this can be written as 4 n + 2 with n = 1 this ring is aromatic according to Hückel's rule: Highlight all aromatic atoms and bonds in benzene: Answer: | |
Aromatic atoms elemental composition

| count C (carbon) | 6
Quantitative molecular descriptors
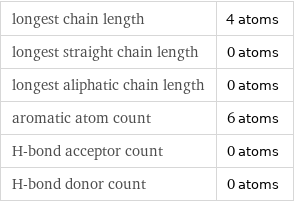
longest chain length | 4 atoms longest straight chain length | 0 atoms longest aliphatic chain length | 0 atoms aromatic atom count | 6 atoms H-bond acceptor count | 0 atoms H-bond donor count | 0 atoms
Corresponding quantity
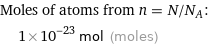
Moles of atoms from n = N/N_A: | 1×10^-23 mol (moles)