Input interpretation
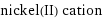
nickel(II) cation
Structure diagram

Structure diagram
General properties

formula | Ni^(2+) net ionic charge | +2 alternate names | nickelous | nickel(II) | nickel(2+)
Ionic radii
nickel(II) cation state | radius 4-coordinate | 49 pm 6-coordinate | 69 pm
Units

Other properties
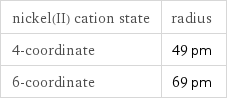
ion class | biomolecule ions | cations | d block ions | group 10 ions | nickel(II) ions | transition metal ions common sources of ion | nickel(II) sulfate hexahydrate (1 eq) | nickel(II) sulfate heptahydrate (1 eq) | nickel(II) sulfamate tetrahydrate (1 eq) | nickel(II) stearate (1 eq) | nickel(II) phthalocyanine (1 eq) | nickel(II) oxalate dihydrate (1 eq) | nickel(II) nitrate hexahydrate (1 eq) | nickel(II) molybdate (1 eq) | nickel(II) bis(4-cyclohexylbutyrate) (1 eq) | nickel chromium oxide (1 eq)
Thermodynamic properties
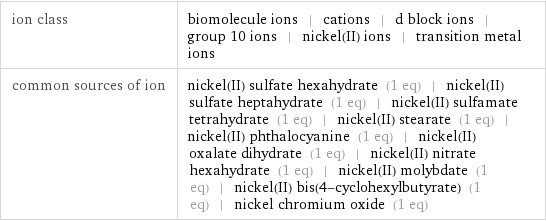
molar free energy of formation Δ_fG° | aqueous | -45.6 kJ/mol (kilojoules per mole) molar heat of formation Δ_fH° | aqueous | -54 kJ/mol (kilojoules per mole) molar entropy S° | aqueous | -128.9 J/(mol K) (joules per mole kelvin)