Input interpretation
nickel(II) chloride | sodium permanganate
Chemical names and formulas
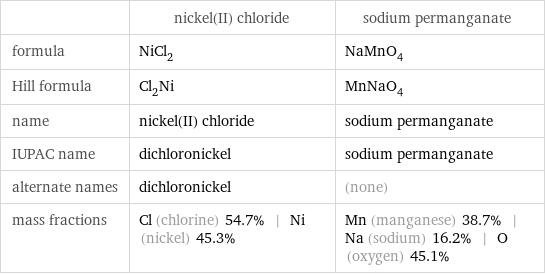
| nickel(II) chloride | sodium permanganate formula | NiCl_2 | NaMnO_4 Hill formula | Cl_2Ni | MnNaO_4 name | nickel(II) chloride | sodium permanganate IUPAC name | dichloronickel | sodium permanganate alternate names | dichloronickel | (none) mass fractions | Cl (chlorine) 54.7% | Ni (nickel) 45.3% | Mn (manganese) 38.7% | Na (sodium) 16.2% | O (oxygen) 45.1%
Structure diagrams
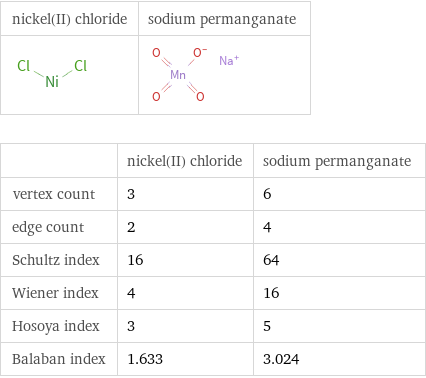
| nickel(II) chloride | sodium permanganate vertex count | 3 | 6 edge count | 2 | 4 Schultz index | 16 | 64 Wiener index | 4 | 16 Hosoya index | 3 | 5 Balaban index | 1.633 | 3.024
Basic properties
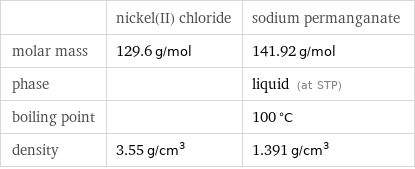
| nickel(II) chloride | sodium permanganate molar mass | 129.6 g/mol | 141.92 g/mol phase | | liquid (at STP) boiling point | | 100 °C density | 3.55 g/cm^3 | 1.391 g/cm^3
Units

Liquid properties

| sodium permanganate density | 1.391 g/cm^3
Units
