Input interpretation
diamminesilver(I) cation | ion class
Result
cations | complex ions | silver(I) ions
Ionic radii
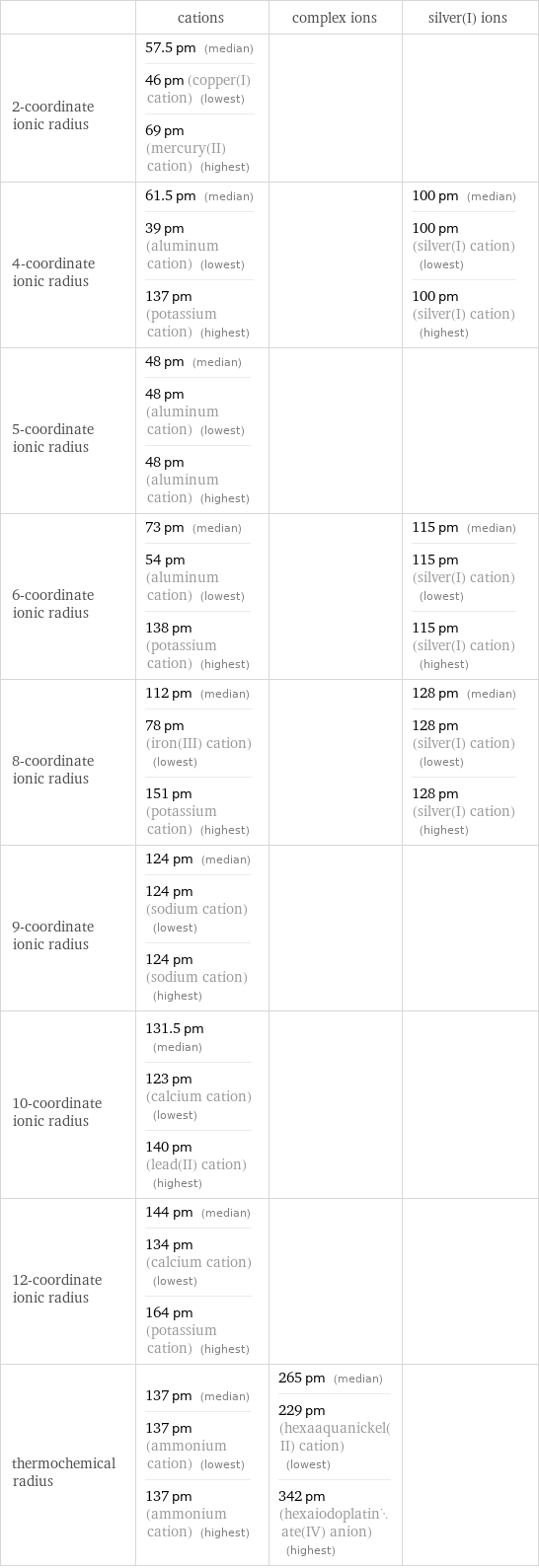
| cations | complex ions | silver(I) ions 2-coordinate ionic radius | 57.5 pm (median) 46 pm (copper(I) cation) (lowest) 69 pm (mercury(II) cation) (highest) | | 4-coordinate ionic radius | 61.5 pm (median) 39 pm (aluminum cation) (lowest) 137 pm (potassium cation) (highest) | | 100 pm (median) 100 pm (silver(I) cation) (lowest) 100 pm (silver(I) cation) (highest) 5-coordinate ionic radius | 48 pm (median) 48 pm (aluminum cation) (lowest) 48 pm (aluminum cation) (highest) | | 6-coordinate ionic radius | 73 pm (median) 54 pm (aluminum cation) (lowest) 138 pm (potassium cation) (highest) | | 115 pm (median) 115 pm (silver(I) cation) (lowest) 115 pm (silver(I) cation) (highest) 8-coordinate ionic radius | 112 pm (median) 78 pm (iron(III) cation) (lowest) 151 pm (potassium cation) (highest) | | 128 pm (median) 128 pm (silver(I) cation) (lowest) 128 pm (silver(I) cation) (highest) 9-coordinate ionic radius | 124 pm (median) 124 pm (sodium cation) (lowest) 124 pm (sodium cation) (highest) | | 10-coordinate ionic radius | 131.5 pm (median) 123 pm (calcium cation) (lowest) 140 pm (lead(II) cation) (highest) | | 12-coordinate ionic radius | 144 pm (median) 134 pm (calcium cation) (lowest) 164 pm (potassium cation) (highest) | | thermochemical radius | 137 pm (median) 137 pm (ammonium cation) (lowest) 137 pm (ammonium cation) (highest) | 265 pm (median) 229 pm (hexaaquanickel(II) cation) (lowest) 342 pm (hexaiodoplatinate(IV) anion) (highest) |