Input interpretation
rubidium chloride | aluminum
Chemical names and formulas
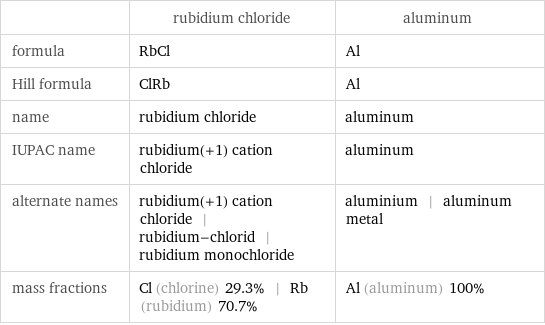
| rubidium chloride | aluminum formula | RbCl | Al Hill formula | ClRb | Al name | rubidium chloride | aluminum IUPAC name | rubidium(+1) cation chloride | aluminum alternate names | rubidium(+1) cation chloride | rubidium-chlorid | rubidium monochloride | aluminium | aluminum metal mass fractions | Cl (chlorine) 29.3% | Rb (rubidium) 70.7% | Al (aluminum) 100%
Structure diagrams

Structure diagrams
Basic properties
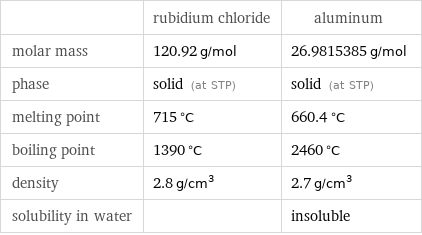
| rubidium chloride | aluminum molar mass | 120.92 g/mol | 26.9815385 g/mol phase | solid (at STP) | solid (at STP) melting point | 715 °C | 660.4 °C boiling point | 1390 °C | 2460 °C density | 2.8 g/cm^3 | 2.7 g/cm^3 solubility in water | | insoluble
Units
