Input interpretation
magnesium carbonate
Chemical names and formulas
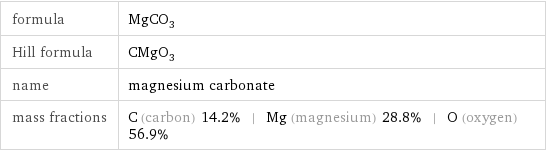
formula | MgCO_3 Hill formula | CMgO_3 name | magnesium carbonate mass fractions | C (carbon) 14.2% | Mg (magnesium) 28.8% | O (oxygen) 56.9%
Structure diagram
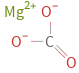
Structure diagram
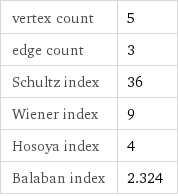
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties

molar mass | 84.313 g/mol
Units

Thermodynamic properties
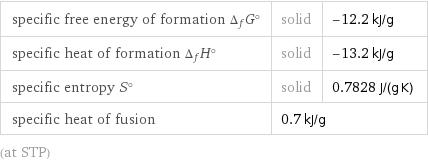
specific free energy of formation Δ_fG° | solid | -12.2 kJ/g specific heat of formation Δ_fH° | solid | -13.2 kJ/g specific entropy S° | solid | 0.7828 J/(g K) specific heat of fusion | 0.7 kJ/g | (at STP)
Units

Chemical identifiers
![CAS number | 546-93-0 PubChem CID number | 11029 SMILES identifier | C(=O)([O-])[O-].[Mg+2]](../image_source/0b4f2311d0c7c6402b7dd4238b4d1701.png)
CAS number | 546-93-0 PubChem CID number | 11029 SMILES identifier | C(=O)([O-])[O-].[Mg+2]
Ion equivalents

Mg^(2+) (magnesium cation) | 1 (CO_3)^(2-) (carbonate anion) | 1