Input interpretation
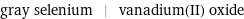
gray selenium | vanadium(II) oxide
Chemical names and formulas
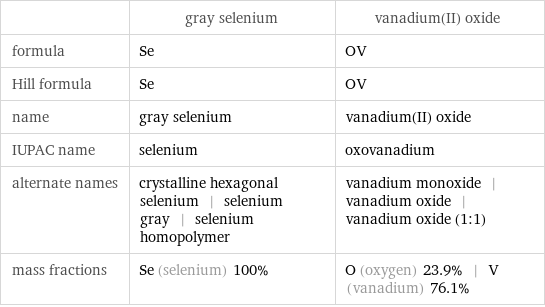
| gray selenium | vanadium(II) oxide formula | Se | OV Hill formula | Se | OV name | gray selenium | vanadium(II) oxide IUPAC name | selenium | oxovanadium alternate names | crystalline hexagonal selenium | selenium gray | selenium homopolymer | vanadium monoxide | vanadium oxide | vanadium oxide (1:1) mass fractions | Se (selenium) 100% | O (oxygen) 23.9% | V (vanadium) 76.1%
Structure diagrams

Structure diagrams
Basic properties
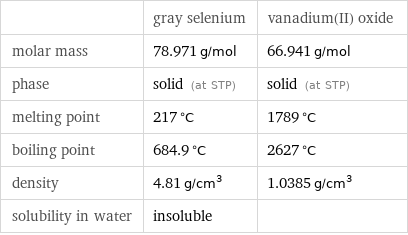
| gray selenium | vanadium(II) oxide molar mass | 78.971 g/mol | 66.941 g/mol phase | solid (at STP) | solid (at STP) melting point | 217 °C | 1789 °C boiling point | 684.9 °C | 2627 °C density | 4.81 g/cm^3 | 1.0385 g/cm^3 solubility in water | insoluble |
Units

Thermodynamic properties
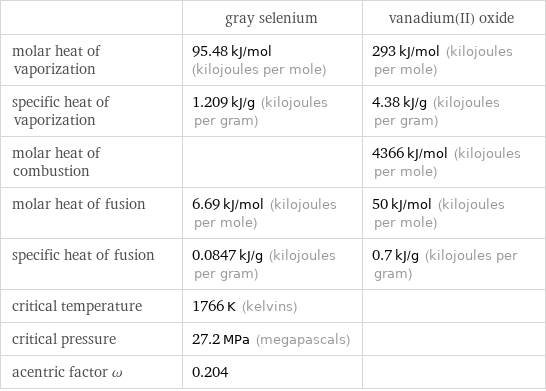
| gray selenium | vanadium(II) oxide molar heat of vaporization | 95.48 kJ/mol (kilojoules per mole) | 293 kJ/mol (kilojoules per mole) specific heat of vaporization | 1.209 kJ/g (kilojoules per gram) | 4.38 kJ/g (kilojoules per gram) molar heat of combustion | | 4366 kJ/mol (kilojoules per mole) molar heat of fusion | 6.69 kJ/mol (kilojoules per mole) | 50 kJ/mol (kilojoules per mole) specific heat of fusion | 0.0847 kJ/g (kilojoules per gram) | 0.7 kJ/g (kilojoules per gram) critical temperature | 1766 K (kelvins) | critical pressure | 27.2 MPa (megapascals) | acentric factor ω | 0.204 |
Solid properties (at STP)
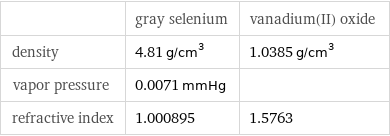
| gray selenium | vanadium(II) oxide density | 4.81 g/cm^3 | 1.0385 g/cm^3 vapor pressure | 0.0071 mmHg | refractive index | 1.000895 | 1.5763
Units

Chemical identifiers
![| gray selenium | vanadium(II) oxide CAS number | 7782-49-2 | 12035-98-2 PubChem CID number | 6326970 | 24411 PubChem SID number | 24852251 | SMILES identifier | [Se] | O=[V] InChI identifier | InChI=1/Se | InChI=1/O.V/rOV/c1-2 EU number | | 234-834-3 Gmelin number | | 13746 RTECS number | VS7700000 | MDL number | MFCD00134090 |](../image_source/73a1cc8f74a6c5db1c6cebd779087c83.png)
| gray selenium | vanadium(II) oxide CAS number | 7782-49-2 | 12035-98-2 PubChem CID number | 6326970 | 24411 PubChem SID number | 24852251 | SMILES identifier | [Se] | O=[V] InChI identifier | InChI=1/Se | InChI=1/O.V/rOV/c1-2 EU number | | 234-834-3 Gmelin number | | 13746 RTECS number | VS7700000 | MDL number | MFCD00134090 |