Input interpretation

polar solvents | molar mass
Summary
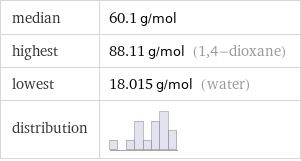
median | 60.1 g/mol highest | 88.11 g/mol (1, 4-dioxane) lowest | 18.015 g/mol (water) distribution |
Units

Distribution plots
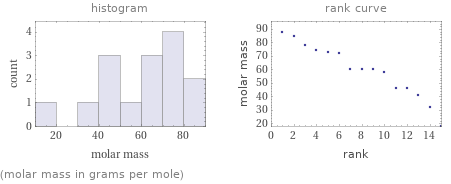
(molar mass in grams per mole)
Molar mass rankings
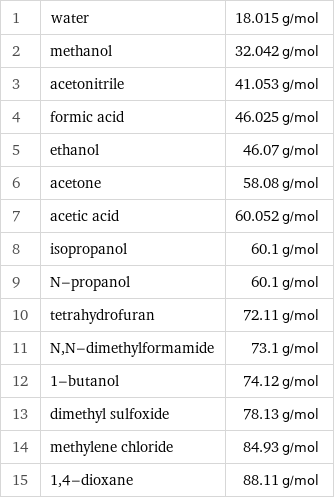
1 | water | 18.015 g/mol 2 | methanol | 32.042 g/mol 3 | acetonitrile | 41.053 g/mol 4 | formic acid | 46.025 g/mol 5 | ethanol | 46.07 g/mol 6 | acetone | 58.08 g/mol 7 | acetic acid | 60.052 g/mol 8 | isopropanol | 60.1 g/mol 9 | N-propanol | 60.1 g/mol 10 | tetrahydrofuran | 72.11 g/mol 11 | N, N-dimethylformamide | 73.1 g/mol 12 | 1-butanol | 74.12 g/mol 13 | dimethyl sulfoxide | 78.13 g/mol 14 | methylene chloride | 84.93 g/mol 15 | 1, 4-dioxane | 88.11 g/mol
Unit conversion for median molar mass 60.1 g/mol
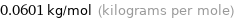
0.0601 kg/mol (kilograms per mole)
Comparisons for median molar mass 60.1 g/mol
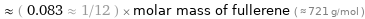
≈ ( 0.083 ≈ 1/12 ) × molar mass of fullerene ( ≈ 721 g/mol )
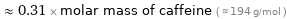
≈ 0.31 × molar mass of caffeine ( ≈ 194 g/mol )
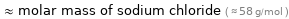
≈ molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
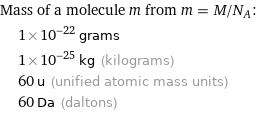
Mass of a molecule m from m = M/N_A: | 1×10^-22 grams | 1×10^-25 kg (kilograms) | 60 u (unified atomic mass units) | 60 Da (daltons)
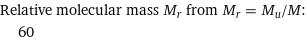
Relative molecular mass M_r from M_r = M_u/M: | 60