Input interpretation
sulfur (chemical element) | oxygen (chemical element) | selenium (chemical element) | chromium (chemical element)
Periodic table location
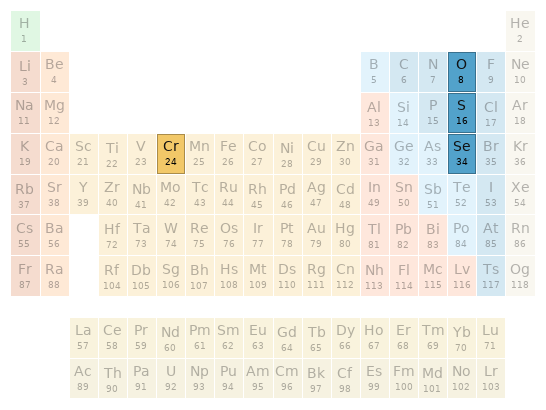
Periodic table location
Images
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration sulfur | S | 16 | [Ne]3s^23p^4 oxygen | O | 8 | [He]2s^22p^4 selenium | Se | 34 | [Ar]4s^23d^104p^4 chromium | Cr | 24 | [Ar]4s^13d^5 | Aufbau diagram | block | group sulfur | 3p 3s | p | 16 oxygen | 2p 2s | p | 16 selenium | 4p 3d 4s | p | 16 chromium | 3d 4s | d | 6 | period | atomic mass | half-life sulfur | 3 | 32.06 u | (stable) oxygen | 2 | 15.999 u | (stable) selenium | 4 | 78.971 u | (stable) chromium | 4 | 51.9961 u | (stable)](../image_source/1ba32781eee2858d59489765ea47c89f.png)
| atomic symbol | atomic number | short electronic configuration sulfur | S | 16 | [Ne]3s^23p^4 oxygen | O | 8 | [He]2s^22p^4 selenium | Se | 34 | [Ar]4s^23d^104p^4 chromium | Cr | 24 | [Ar]4s^13d^5 | Aufbau diagram | block | group sulfur | 3p 3s | p | 16 oxygen | 2p 2s | p | 16 selenium | 4p 3d 4s | p | 16 chromium | 3d 4s | d | 6 | period | atomic mass | half-life sulfur | 3 | 32.06 u | (stable) oxygen | 2 | 15.999 u | (stable) selenium | 4 | 78.971 u | (stable) chromium | 4 | 51.9961 u | (stable)
Thermodynamic properties
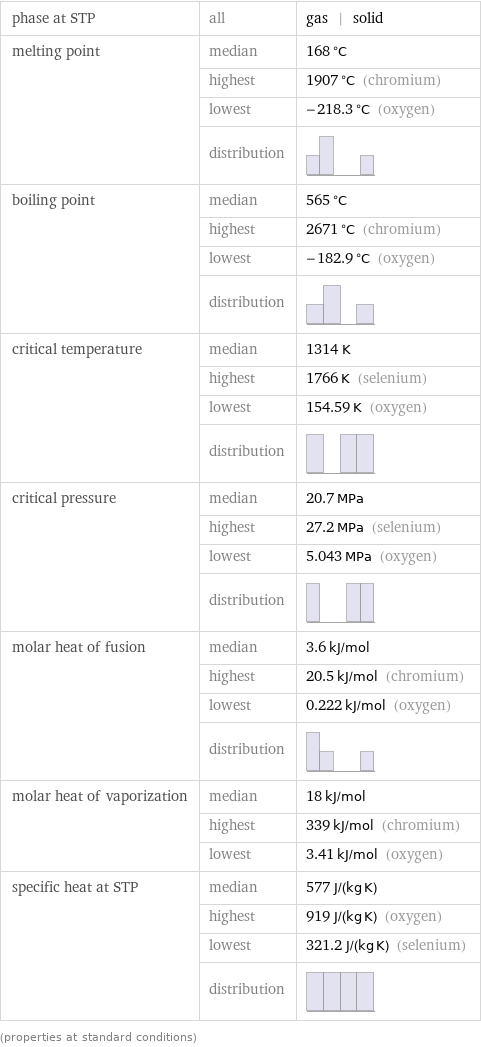
phase at STP | all | gas | solid melting point | median | 168 °C | highest | 1907 °C (chromium) | lowest | -218.3 °C (oxygen) | distribution | boiling point | median | 565 °C | highest | 2671 °C (chromium) | lowest | -182.9 °C (oxygen) | distribution | critical temperature | median | 1314 K | highest | 1766 K (selenium) | lowest | 154.59 K (oxygen) | distribution | critical pressure | median | 20.7 MPa | highest | 27.2 MPa (selenium) | lowest | 5.043 MPa (oxygen) | distribution | molar heat of fusion | median | 3.6 kJ/mol | highest | 20.5 kJ/mol (chromium) | lowest | 0.222 kJ/mol (oxygen) | distribution | molar heat of vaporization | median | 18 kJ/mol | highest | 339 kJ/mol (chromium) | lowest | 3.41 kJ/mol (oxygen) specific heat at STP | median | 577 J/(kg K) | highest | 919 J/(kg K) (oxygen) | lowest | 321.2 J/(kg K) (selenium) | distribution | (properties at standard conditions)
Material properties
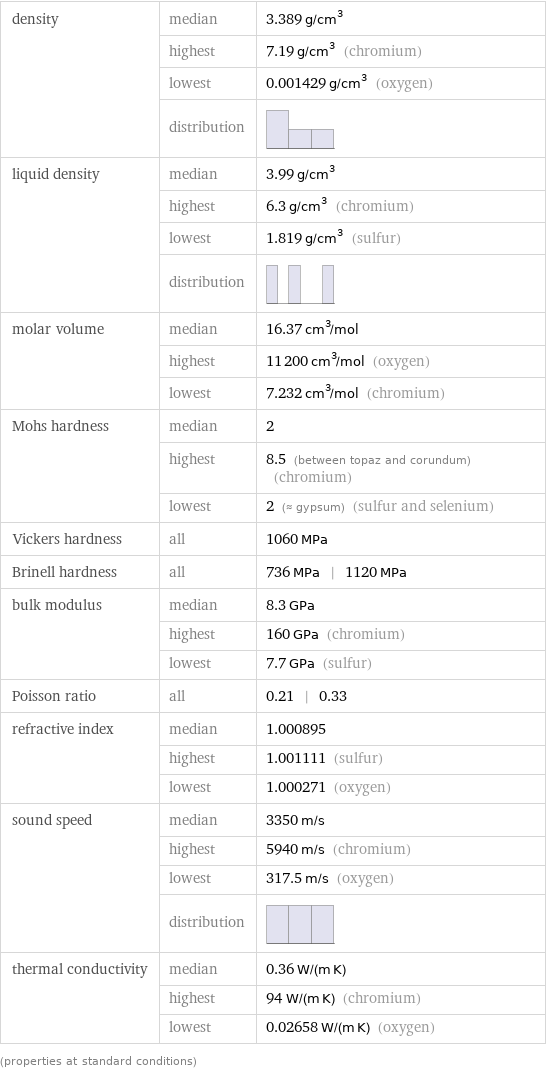
density | median | 3.389 g/cm^3 | highest | 7.19 g/cm^3 (chromium) | lowest | 0.001429 g/cm^3 (oxygen) | distribution | liquid density | median | 3.99 g/cm^3 | highest | 6.3 g/cm^3 (chromium) | lowest | 1.819 g/cm^3 (sulfur) | distribution | molar volume | median | 16.37 cm^3/mol | highest | 11200 cm^3/mol (oxygen) | lowest | 7.232 cm^3/mol (chromium) Mohs hardness | median | 2 | highest | 8.5 (between topaz and corundum) (chromium) | lowest | 2 (≈ gypsum) (sulfur and selenium) Vickers hardness | all | 1060 MPa Brinell hardness | all | 736 MPa | 1120 MPa bulk modulus | median | 8.3 GPa | highest | 160 GPa (chromium) | lowest | 7.7 GPa (sulfur) Poisson ratio | all | 0.21 | 0.33 refractive index | median | 1.000895 | highest | 1.001111 (sulfur) | lowest | 1.000271 (oxygen) sound speed | median | 3350 m/s | highest | 5940 m/s (chromium) | lowest | 317.5 m/s (oxygen) | distribution | thermal conductivity | median | 0.36 W/(m K) | highest | 94 W/(m K) (chromium) | lowest | 0.02658 W/(m K) (oxygen) (properties at standard conditions)
Reactivity
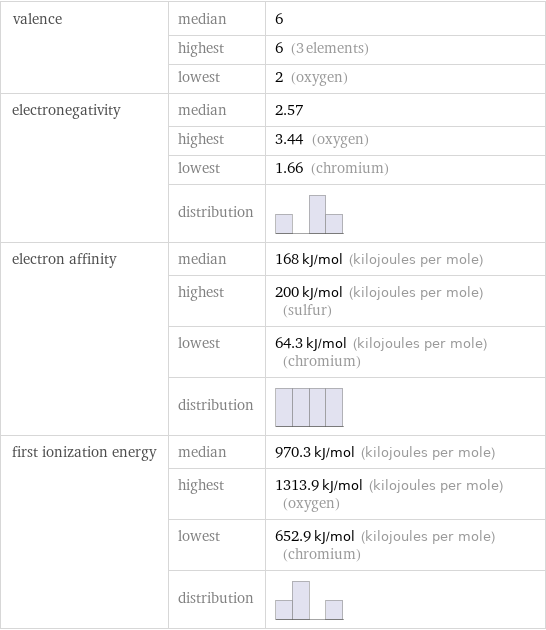
valence | median | 6 | highest | 6 (3 elements) | lowest | 2 (oxygen) electronegativity | median | 2.57 | highest | 3.44 (oxygen) | lowest | 1.66 (chromium) | distribution | electron affinity | median | 168 kJ/mol (kilojoules per mole) | highest | 200 kJ/mol (kilojoules per mole) (sulfur) | lowest | 64.3 kJ/mol (kilojoules per mole) (chromium) | distribution | first ionization energy | median | 970.3 kJ/mol (kilojoules per mole) | highest | 1313.9 kJ/mol (kilojoules per mole) (oxygen) | lowest | 652.9 kJ/mol (kilojoules per mole) (chromium) | distribution |
Atomic properties
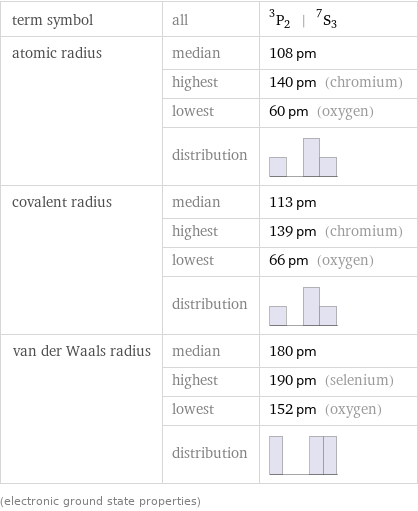
term symbol | all | ^3P_2 | ^7S_3 atomic radius | median | 108 pm | highest | 140 pm (chromium) | lowest | 60 pm (oxygen) | distribution | covalent radius | median | 113 pm | highest | 139 pm (chromium) | lowest | 66 pm (oxygen) | distribution | van der Waals radius | median | 180 pm | highest | 190 pm (selenium) | lowest | 152 pm (oxygen) | distribution | (electronic ground state properties)
Abundances
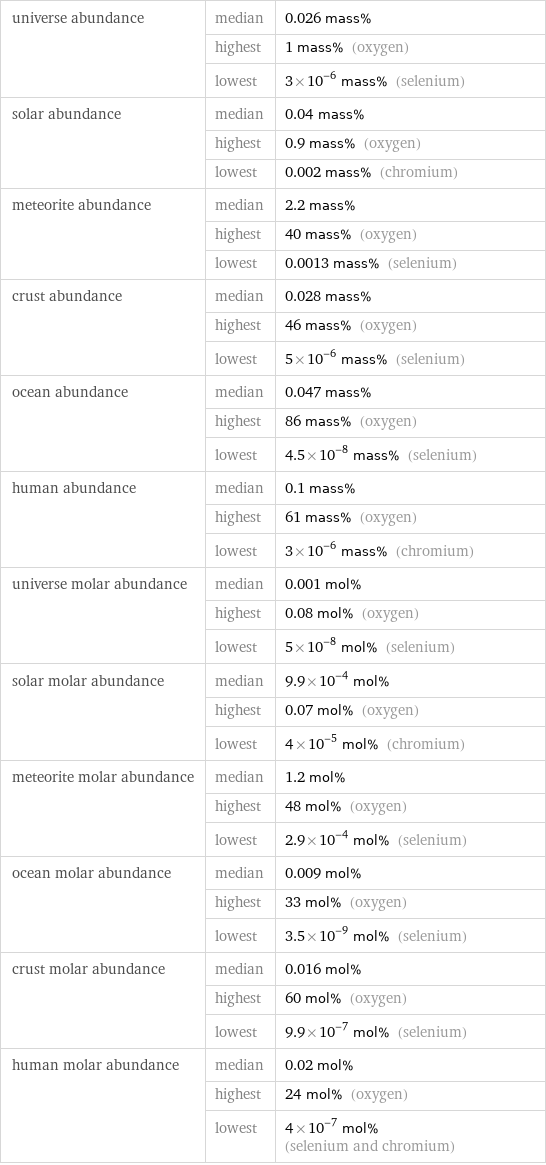
universe abundance | median | 0.026 mass% | highest | 1 mass% (oxygen) | lowest | 3×10^-6 mass% (selenium) solar abundance | median | 0.04 mass% | highest | 0.9 mass% (oxygen) | lowest | 0.002 mass% (chromium) meteorite abundance | median | 2.2 mass% | highest | 40 mass% (oxygen) | lowest | 0.0013 mass% (selenium) crust abundance | median | 0.028 mass% | highest | 46 mass% (oxygen) | lowest | 5×10^-6 mass% (selenium) ocean abundance | median | 0.047 mass% | highest | 86 mass% (oxygen) | lowest | 4.5×10^-8 mass% (selenium) human abundance | median | 0.1 mass% | highest | 61 mass% (oxygen) | lowest | 3×10^-6 mass% (chromium) universe molar abundance | median | 0.001 mol% | highest | 0.08 mol% (oxygen) | lowest | 5×10^-8 mol% (selenium) solar molar abundance | median | 9.9×10^-4 mol% | highest | 0.07 mol% (oxygen) | lowest | 4×10^-5 mol% (chromium) meteorite molar abundance | median | 1.2 mol% | highest | 48 mol% (oxygen) | lowest | 2.9×10^-4 mol% (selenium) ocean molar abundance | median | 0.009 mol% | highest | 33 mol% (oxygen) | lowest | 3.5×10^-9 mol% (selenium) crust molar abundance | median | 0.016 mol% | highest | 60 mol% (oxygen) | lowest | 9.9×10^-7 mol% (selenium) human molar abundance | median | 0.02 mol% | highest | 24 mol% (oxygen) | lowest | 4×10^-7 mol% (selenium and chromium)