Input interpretation
chromium(III) oxide | potassium superoxide
Chemical names and formulas
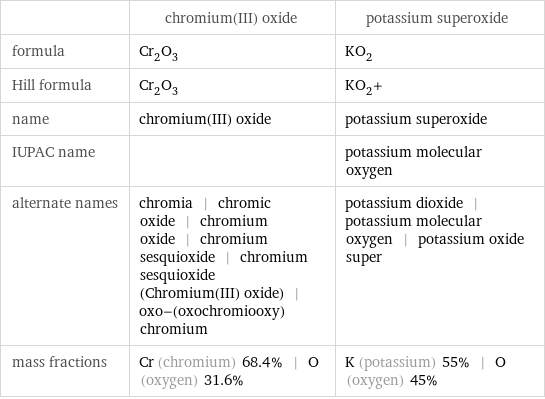
| chromium(III) oxide | potassium superoxide formula | Cr_2O_3 | KO_2 Hill formula | Cr_2O_3 | KO_2+ name | chromium(III) oxide | potassium superoxide IUPAC name | | potassium molecular oxygen alternate names | chromia | chromic oxide | chromium oxide | chromium sesquioxide | chromium sesquioxide (Chromium(III) oxide) | oxo-(oxochromiooxy)chromium | potassium dioxide | potassium molecular oxygen | potassium oxide super mass fractions | Cr (chromium) 68.4% | O (oxygen) 31.6% | K (potassium) 55% | O (oxygen) 45%
Structure diagrams
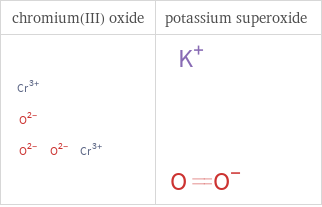
Structure diagrams
Basic properties
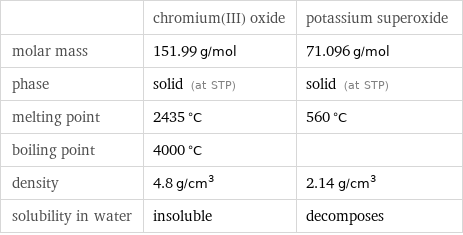
| chromium(III) oxide | potassium superoxide molar mass | 151.99 g/mol | 71.096 g/mol phase | solid (at STP) | solid (at STP) melting point | 2435 °C | 560 °C boiling point | 4000 °C | density | 4.8 g/cm^3 | 2.14 g/cm^3 solubility in water | insoluble | decomposes
Units

Thermodynamic properties
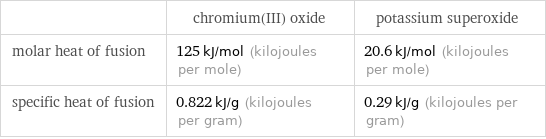
| chromium(III) oxide | potassium superoxide molar heat of fusion | 125 kJ/mol (kilojoules per mole) | 20.6 kJ/mol (kilojoules per mole) specific heat of fusion | 0.822 kJ/g (kilojoules per gram) | 0.29 kJ/g (kilojoules per gram)
Solid properties (at STP)

| chromium(III) oxide | potassium superoxide density | 4.8 g/cm^3 | 2.14 g/cm^3 vapor pressure | 3 mmHg |
Units

Chemical identifiers
![| chromium(III) oxide | potassium superoxide CAS number | 1308-38-9 | 12030-88-5 PubChem CID number | | 61541 PubChem SID number | | 24856841 SMILES identifier | [Cr+3].[Cr+3].[O-2].[O-2].[O-2] | O=O.[K+]](../image_source/c6581d7683ac5f2ab20672d3de7999ab.png)
| chromium(III) oxide | potassium superoxide CAS number | 1308-38-9 | 12030-88-5 PubChem CID number | | 61541 PubChem SID number | | 24856841 SMILES identifier | [Cr+3].[Cr+3].[O-2].[O-2].[O-2] | O=O.[K+]