Input interpretation
aluminum oxide
Chemical names and formulas

formula | Al_2O_3 name | aluminum oxide IUPAC name | dialuminum;oxygen(2-) alternate names | alumina | keto-ketoalumanyloxy-alumane | oxo-oxoalumanyloxy-alumane | oxo-oxoalumanyloxyalumane mass fractions | Al (aluminum) 52.9% | O (oxygen) 47.1%
Structure diagram
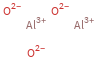
Structure diagram
Basic properties
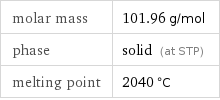
molar mass | 101.96 g/mol phase | solid (at STP) melting point | 2040 °C
Units

Thermodynamic properties

specific free energy of formation Δ_fG° | solid | -15.52 kJ/g molar free energy of formation Δ_fG° | solid | -1582 kJ/mol specific heat of formation Δ_fH° | solid | -16.44 kJ/g molar heat of formation Δ_fH° | solid | -1676 kJ/mol specific entropy S° | solid | 0.5002 J/(g K) molar entropy S° | solid | 51 J/(mol K) molar heat of fusion | 111.1 kJ/mol | specific heat of fusion | 1.09 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1344-28-1 PubChem CID number | 9989226 PubChem SID number | 24852104 SMILES identifier | [O-2].[O-2].[O-2].[Al+3].[Al+3] InChI identifier | InChI=1/2Al.3O/rAl2O3/c3-1-5-2-4 RTECS number | BD1200000 MDL number | MFCD00003424](../image_source/b19650ed3971fc1cce8e86952ee471b1.png)
CAS number | 1344-28-1 PubChem CID number | 9989226 PubChem SID number | 24852104 SMILES identifier | [O-2].[O-2].[O-2].[Al+3].[Al+3] InChI identifier | InChI=1/2Al.3O/rAl2O3/c3-1-5-2-4 RTECS number | BD1200000 MDL number | MFCD00003424
Toxicity properties

odor | odorless short-term exposure limit | 20 mg/m^3

long-term exposure limit | 10 mg/m^3 (over 8 hours)