Input interpretation
potassium aminetrichloroplatinate(II) | lithium amide
Chemical names and formulas
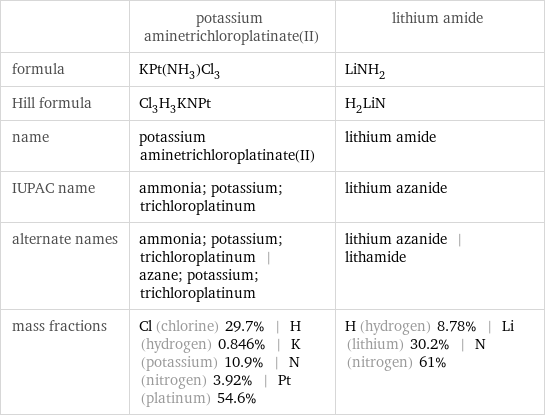
| potassium aminetrichloroplatinate(II) | lithium amide formula | KPt(NH_3)Cl_3 | LiNH_2 Hill formula | Cl_3H_3KNPt | H_2LiN name | potassium aminetrichloroplatinate(II) | lithium amide IUPAC name | ammonia; potassium; trichloroplatinum | lithium azanide alternate names | ammonia; potassium; trichloroplatinum | azane; potassium; trichloroplatinum | lithium azanide | lithamide mass fractions | Cl (chlorine) 29.7% | H (hydrogen) 0.846% | K (potassium) 10.9% | N (nitrogen) 3.92% | Pt (platinum) 54.6% | H (hydrogen) 8.78% | Li (lithium) 30.2% | N (nitrogen) 61%
Structure diagrams
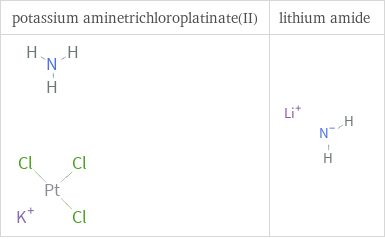
Structure diagrams
Basic properties
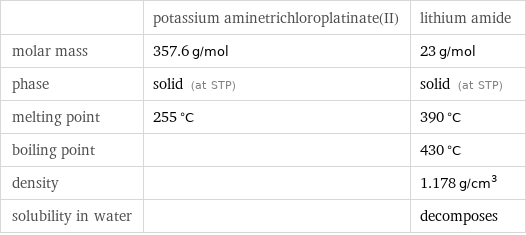
| potassium aminetrichloroplatinate(II) | lithium amide molar mass | 357.6 g/mol | 23 g/mol phase | solid (at STP) | solid (at STP) melting point | 255 °C | 390 °C boiling point | | 430 °C density | | 1.178 g/cm^3 solubility in water | | decomposes
Units

Solid properties (at STP)
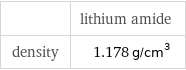
| lithium amide density | 1.178 g/cm^3
Units
