Input interpretation

insulators | number of valence electrons
Summary
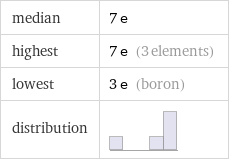
median | 7 e highest | 7 e (3 elements) lowest | 3 e (boron) distribution |
Units

Ranked values
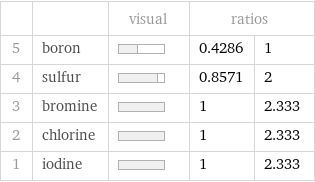
| | visual | ratios | 5 | boron | | 0.4286 | 1 4 | sulfur | | 0.8571 | 2 3 | bromine | | 1 | 2.333 2 | chlorine | | 1 | 2.333 1 | iodine | | 1 | 2.333
Number of valence electrons rankings
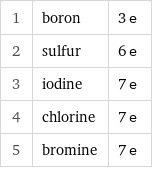
1 | boron | 3 e 2 | sulfur | 6 e 3 | iodine | 7 e 4 | chlorine | 7 e 5 | bromine | 7 e
Corresponding quantity
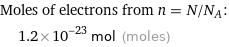
Moles of electrons from n = N/N_A: | 1.2×10^-23 mol (moles)