Input interpretation
hydrogen chloride | iodine bromide
Chemical names and formulas
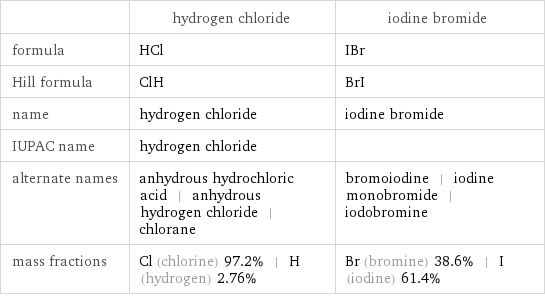
| hydrogen chloride | iodine bromide formula | HCl | IBr Hill formula | ClH | BrI name | hydrogen chloride | iodine bromide IUPAC name | hydrogen chloride | alternate names | anhydrous hydrochloric acid | anhydrous hydrogen chloride | chlorane | bromoiodine | iodine monobromide | iodobromine mass fractions | Cl (chlorine) 97.2% | H (hydrogen) 2.76% | Br (bromine) 38.6% | I (iodine) 61.4%
Structure diagrams
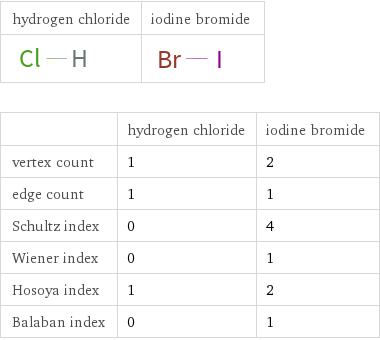
| hydrogen chloride | iodine bromide vertex count | 1 | 2 edge count | 1 | 1 Schultz index | 0 | 4 Wiener index | 0 | 1 Hosoya index | 1 | 2 Balaban index | 0 | 1
3D structure
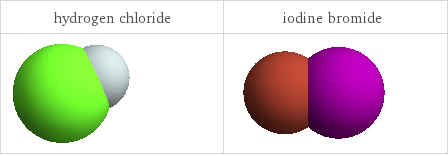
3D structure
Basic properties
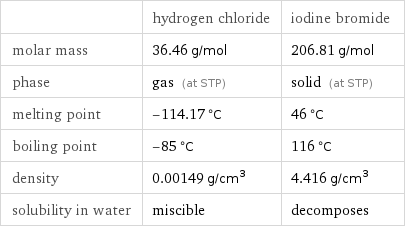
| hydrogen chloride | iodine bromide molar mass | 36.46 g/mol | 206.81 g/mol phase | gas (at STP) | solid (at STP) melting point | -114.17 °C | 46 °C boiling point | -85 °C | 116 °C density | 0.00149 g/cm^3 | 4.416 g/cm^3 solubility in water | miscible | decomposes
Units

Gas properties
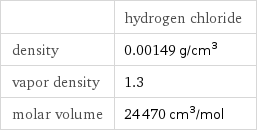
| hydrogen chloride density | 0.00149 g/cm^3 vapor density | 1.3 molar volume | 24470 cm^3/mol
Units

Liquid properties

| hydrogen chloride sound speed | 1066 km/h
Units

Thermodynamic properties
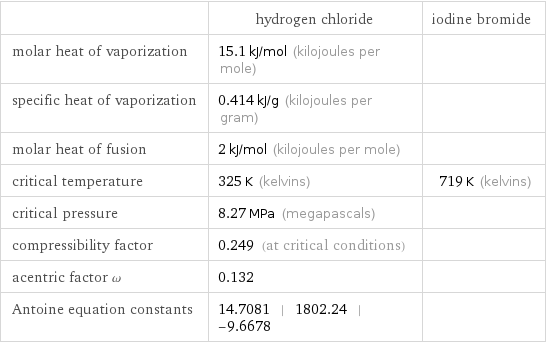
| hydrogen chloride | iodine bromide molar heat of vaporization | 15.1 kJ/mol (kilojoules per mole) | specific heat of vaporization | 0.414 kJ/g (kilojoules per gram) | molar heat of fusion | 2 kJ/mol (kilojoules per mole) | critical temperature | 325 K (kelvins) | 719 K (kelvins) critical pressure | 8.27 MPa (megapascals) | compressibility factor | 0.249 (at critical conditions) | acentric factor ω | 0.132 | Antoine equation constants | 14.7081 | 1802.24 | -9.6678 |
Solid properties
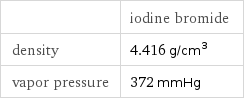
| iodine bromide density | 4.416 g/cm^3 vapor pressure | 372 mmHg
Units
