Input interpretation
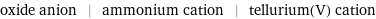
oxide anion | ammonium cation | tellurium(V) cation
Structure diagrams
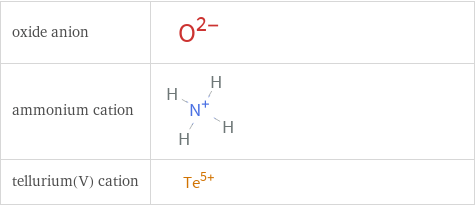
Structure diagrams
General properties
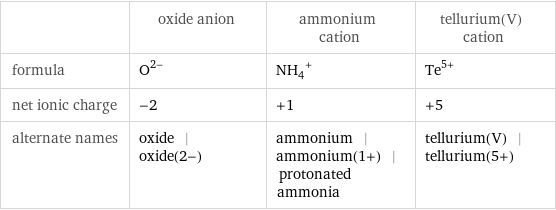
| oxide anion | ammonium cation | tellurium(V) cation formula | O^(2-) | (NH_4)^+ | Te^(5+) net ionic charge | -2 | +1 | +5 alternate names | oxide | oxide(2-) | ammonium | ammonium(1+) | protonated ammonia | tellurium(V) | tellurium(5+)
Ionic radii
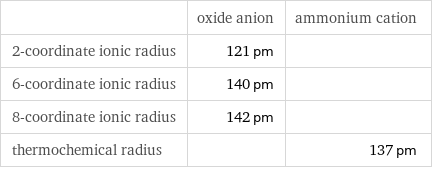
| oxide anion | ammonium cation 2-coordinate ionic radius | 121 pm | 6-coordinate ionic radius | 140 pm | 8-coordinate ionic radius | 142 pm | thermochemical radius | | 137 pm
Units

Other properties
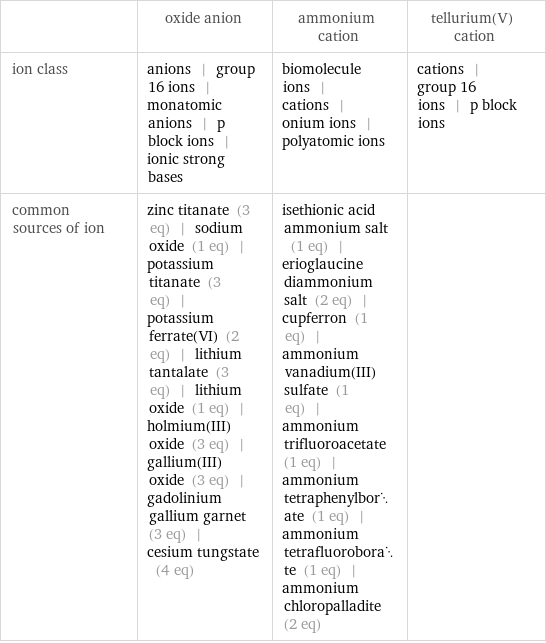
| oxide anion | ammonium cation | tellurium(V) cation ion class | anions | group 16 ions | monatomic anions | p block ions | ionic strong bases | biomolecule ions | cations | onium ions | polyatomic ions | cations | group 16 ions | p block ions common sources of ion | zinc titanate (3 eq) | sodium oxide (1 eq) | potassium titanate (3 eq) | potassium ferrate(VI) (2 eq) | lithium tantalate (3 eq) | lithium oxide (1 eq) | holmium(III) oxide (3 eq) | gallium(III) oxide (3 eq) | gadolinium gallium garnet (3 eq) | cesium tungstate (4 eq) | isethionic acid ammonium salt (1 eq) | erioglaucine diammonium salt (2 eq) | cupferron (1 eq) | ammonium vanadium(III) sulfate (1 eq) | ammonium trifluoroacetate (1 eq) | ammonium tetraphenylborate (1 eq) | ammonium tetrafluoroborate (1 eq) | ammonium chloropalladite (2 eq) |