Input interpretation
lithium fluoride
Chemical names and formulas
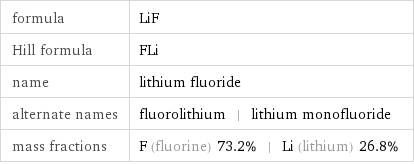
formula | LiF Hill formula | FLi name | lithium fluoride alternate names | fluorolithium | lithium monofluoride mass fractions | F (fluorine) 73.2% | Li (lithium) 26.8%
Structure diagram

Structure diagram
Basic properties
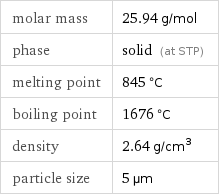
molar mass | 25.94 g/mol phase | solid (at STP) melting point | 845 °C boiling point | 1676 °C density | 2.64 g/cm^3 particle size | 5 µm
Solid properties (at STP)
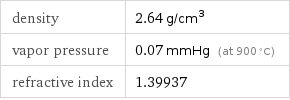
density | 2.64 g/cm^3 vapor pressure | 0.07 mmHg (at 900 °C) refractive index | 1.39937
Units

Thermodynamic properties
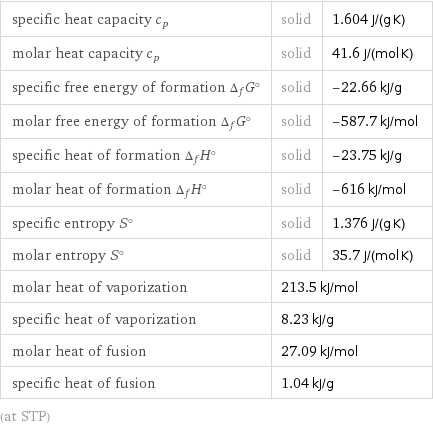
specific heat capacity c_p | solid | 1.604 J/(g K) molar heat capacity c_p | solid | 41.6 J/(mol K) specific free energy of formation Δ_fG° | solid | -22.66 kJ/g molar free energy of formation Δ_fG° | solid | -587.7 kJ/mol specific heat of formation Δ_fH° | solid | -23.75 kJ/g molar heat of formation Δ_fH° | solid | -616 kJ/mol specific entropy S° | solid | 1.376 J/(g K) molar entropy S° | solid | 35.7 J/(mol K) molar heat of vaporization | 213.5 kJ/mol | specific heat of vaporization | 8.23 kJ/g | molar heat of fusion | 27.09 kJ/mol | specific heat of fusion | 1.04 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7789-24-4 PubChem CID number | 224478 PubChem SID number | 24852188 SMILES identifier | [Li+].[F-] InChI identifier | InChI=1/FH.Li/h1H;/q;+1/p-1/fF.Li/h1h;/q-1;m RTECS number | OJ6125000 MDL number | MFCD00011090](../image_source/f9fdbccefe693269e57f42747db1a2dc.png)
CAS number | 7789-24-4 PubChem CID number | 224478 PubChem SID number | 24852188 SMILES identifier | [Li+].[F-] InChI identifier | InChI=1/FH.Li/h1H;/q;+1/p-1/fF.Li/h1h;/q-1;m RTECS number | OJ6125000 MDL number | MFCD00011090
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | other
Ion equivalents

Li^+ (lithium cation) | 1 F^- (fluoride anion) | 1