Input interpretation

potassium bromate | zinc fluoride
Chemical names and formulas
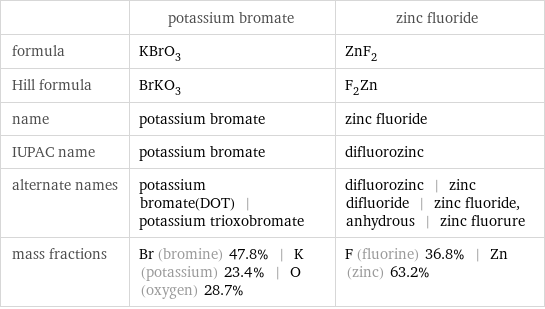
| potassium bromate | zinc fluoride formula | KBrO_3 | ZnF_2 Hill formula | BrKO_3 | F_2Zn name | potassium bromate | zinc fluoride IUPAC name | potassium bromate | difluorozinc alternate names | potassium bromate(DOT) | potassium trioxobromate | difluorozinc | zinc difluoride | zinc fluoride, anhydrous | zinc fluorure mass fractions | Br (bromine) 47.8% | K (potassium) 23.4% | O (oxygen) 28.7% | F (fluorine) 36.8% | Zn (zinc) 63.2%
Structure diagrams
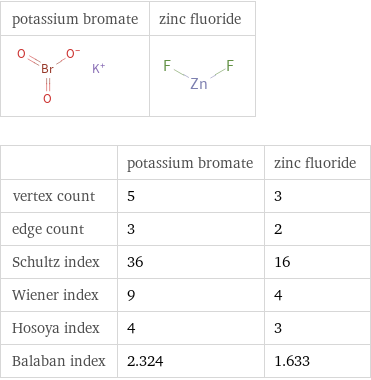
| potassium bromate | zinc fluoride vertex count | 5 | 3 edge count | 3 | 2 Schultz index | 36 | 16 Wiener index | 9 | 4 Hosoya index | 4 | 3 Balaban index | 2.324 | 1.633
Basic properties
| potassium bromate | zinc fluoride molar mass | 167 g/mol | 103.4 g/mol phase | solid (at STP) | solid (at STP) melting point | 350 °C | 872 °C boiling point | | 1500 °C density | 3.218 g/cm^3 | 4.95 g/cm^3
Units

Thermodynamic properties
| zinc fluoride molar heat of vaporization | 230.1 kJ/mol (kilojoules per mole) specific heat of vaporization | 2.226 kJ/g (kilojoules per gram) molar heat of fusion | 40 kJ/mol (kilojoules per mole)
Solid properties (at STP)

| potassium bromate | zinc fluoride density | 3.218 g/cm^3 | 4.95 g/cm^3 vapor pressure | | 0.9998 mmHg
Units

Chemical identifiers
![| potassium bromate | zinc fluoride CAS number | 7758-01-2 | 7783-49-5 PubChem CID number | 24444 | 24551 PubChem SID number | | 24852207 SMILES identifier | [O-]Br(=O)=O.[K+] | F[Zn]F](../image_source/1697c9bb1f7d6d28f9de8aba77cf6c74.png)
| potassium bromate | zinc fluoride CAS number | 7758-01-2 | 7783-49-5 PubChem CID number | 24444 | 24551 PubChem SID number | | 24852207 SMILES identifier | [O-]Br(=O)=O.[K+] | F[Zn]F
NFPA label

NFPA label